Abstract
The aim of this study was to determine the effect of dietary Forsythia suspensa extract (FSE) supplementation to lactating sows and nursery pigs on post-weaning performance, antioxidant capacity, immunoglobulins, and intestinal health. Based on backfat, body weight (BW), and parity, 24 gestating sows (Landrace × Yorkshire) with average parity of 3.38 ± 0.61 and BW of 234 ± 6.81 kg were allotted into two dietary treatments (control vs. 100 mg/kg FSE) with 12 sows per treatment from day 107 of gestation to day 21 of lactation. After weaning, based on the initial BW and source litter, 192 nursery pigs (Duroc × [Landrace × Yorkshire], average BW of 6.98 ± 0.32 kg, weaned at day 21) were allotted into four dietary treatments with eight replicate pens per treatment, six pigs per pen for a 4-wk study. The treatments included the following: 1) CC (sows and their piglets both fed control diet); 2) CF (sows fed control diet and their piglets fed FSE diet [containing 100 mg/kg FSE]); 3) FC (sows fed FSE diet and their piglets fed control diet); and 4) FF (sows and their piglets both fed FSE diet). The MIXED procedures of SAS for a split-plot arrangement with sow diet as the whole plot and nursery diet as split plot were used to analyze the data. After weaning, piglets from FSE-fed sows had improved (P < 0.05) average daily gain and feed efficiency, and lower (P < 0.05) diarrhea rate in overall (day 1 to 28) compared with those from sows fed control diet. Piglets from FSE-fed sows also had higher (P < 0.05) contents of immunoglobulin G (IgG), growth hormone, superoxide dismutase (SOD), total antioxidant capacity in serum, villus height in ileum, and villus height to crypt depth ratio in jejunum, as well as lower (P < 0.05) content of malondialdehyde (MDA) in serum and crypt depth in ileum compared with those from sows fed control diet. Piglets fed FSE during nursery had increased (P < 0.05) concentrations of IgG, SOD, and catalase, and decreased (P < 0.05) MDA and tumor nuclear factor-α levels in serum compared with those fed control diet during nursery. Piglets from FC group had increased (P < 0.05) protein expression of occludin in jejunal mucosa and relative abundance of Lactobacillus on genus level in colon compared with those from CC group. In conclusion, for the performance and intestinal health, diets supplemented with FSE during lactation phase seemed more efficient to alleviate weaning stress than the nursery phase. In terms of the antioxidant status and immunoglobulins, FSE supplemented in both phases were efficient for nursery pigs.
Keywords: antioxidant capacity, Forsythia suspensa extract, immunoglobulins, intestinal morphology, nursery pigs, performance
Introduction
For the growth and development of pigs, the lactation and the first 2 wk of post-weaning are critical periods (Wu et al., 2014). Several approaches have been used for improving the intestinal health of post-weaning pigs, for the reason that piglets are easily faced with weaning stress during weaning transition, which mainly causes physiological and morphological changes, gut dysfunction, reduced feed intake, and poor growth performance (Pluske et al., 1997; Lallès et al., 2004; Yin et al., 2013). Traditionally, post-weaning manipulations in diet of nursery pigs are the common ways to alleviate weaning-induced gut dysfunction (Boudry et al., 2004), while previous studies in our laboratory have demonstrated that supplementation with organic acid (Long et al., 2018), essential oils (Zeng et al., 2014), and herb extract (Long et al., 2019) in diet of nursery pigs could effectively alleviate weaning stress and improve their antioxidant status, immunity, and performance. Kim et al. (2013) pointed out the maternal dietary antioxidant supplementation in sows seemed to help on preventing excessive oxidative stress, which still needed to be re-evaluated. Recent studies have showed that maternal diet supplemented with live yeast or seaweed extract in sows during gestation and lactation seemed also a reliable way to alleviate weaning stress or enhance post-weaning growth performance in their offsprings (Leonard et al., 2010, 2011, 2012; Hang et al., 2019), which indicated a novel strategy to alleviate weaning stress.
Forsythia suspensa extract (FSE) is a traditional herb extract; previous studies of our laboratory have demonstrated that FSE was effective in improving antioxidant capacities in vivo (Wang et al., 2008; Long et al., 2020) and in vitro (Lu et al., 2010), and the main antioxidant functional compounds were forsythiaside A, forythialan A, phillyrin, and phillygenin (Lu et al., 2010). Furthermore, FSE could also alleviate high stocking density-induced (Zhang et al., 2013), corticosterone-induced (Zeng et al., 2014), LPS-induced (Zhao et al., 2017b), and transport-induced (Pan et al., 2018a) oxidative stresses in broilers or nursery pigs. Moreover, FSE could also increase nutrient digestibility, immune function, and thus improve the performance in nursery pigs (Zhao et al., 2017a; Long et al., 2019). We also found dietary supplementation with FSE could effectively modulate intestinal morphology and microbiota community in broilers or nursery pigs (Han et al., 2012; Long et al., 2019). Besides, diet supplemented with FSE in late gestating sows could increase the nutrient utilization, antioxidant status, and inflammatory responses, and eventually improve reproductive performance of sows during farrowing (Long et al., 2021). Based on previous studies, we hypothesized that maternal diet supplemented with FSE in sows could alleviate weaning stress or enhance post-weaning performance and gut health in their offsprings. Therefore, the objective of this study was to investigate the effects of dietary FSE supplementation to lactating sows and nursery pigs on post-weaning performance, nutrient digestibility, antioxidant capacity, immunoglobulins, and intestinal health.
Materials and Methods
The Institutional Animal Protection and Use Committee of China Agricultural University (Beijing, China) agreed the procedure and operations used in this experiment (AW09089103-1). The trial was conducted at the Feng Ning Swine Research Unit of China Agricultural University (Chengde, Hebei, China).
Experimental products
According to the procedure by the study of Lu et al. (2010) in our laboratory, 80% ethanol (500 mL) was used to extract the fruits of Forsythia. The ethanol was used to extract the residue twice and then the rotary vaporization (Buchi, Rotavapor R-124, Flawil, Switzerland) was used to dry and combine the filtrates. The major active antioxidant compounds in FSE were forythialan A (82.6 mg/kg), forsythiaside A (33.0 mg/kg), phillyrin (163.4 mg/kg), and phillygenin (33.4 mg/kg).
Animals, diets, and experimental design
Based on backfat, body weight (BW) and parity, 24 sows (Landrace × Yorkshire) with average parity of 3.38 ± 0.61 and BW of 234 ± 6.81 kg were allotted into two dietary treatments (control vs. 100 mg/kg FSE) with 12 sows per treatment. About 1 wk before farrowing, sows were moved to farrowing crates. Newly born piglets were weighed about 48 h after farrowing. Litter size at birth per sow and mortality were recorded. Piglets were weaned at an average of 21 d of age. The birth weight, litter size, weaning weight, and mortality of piglets were measured. The ingredients and nutrient levels in basal diet for lactation sows was shown in Table 1. The nutrient levels met or exceeded the recommended requirement suggested in NRC (2012).
Table 1.
Ingredients and nutrient composition in basal diets of sows (%, as-fed basis)
| Items | Lactation |
|---|---|
| Ingredients | |
| Corn | 62.71 |
| Soybean meal | 23.00 |
| Wheat bran | 8.00 |
| Soybean oil | 3.20 |
| Dicalcium phosphate | 0.80 |
| Limestone | 1.15 |
| Salt | 0.30 |
| L-lysine HCl, 78% | 0.08 |
| L-valine, 99% | 0.26 |
| Vitamin-mineral premix1 | 0.50 |
| Nutrients compositions | |
| Digestible energy, MJ/kg2 | 14.18 |
| Crude protein3 | 17.00 |
| Calcium3 | 0.69 |
| Digestible phosphorus2 | 0.29 |
| Lysine3 | 0.91 |
| Methionine + cysteine3 | 0.57 |
| Threonine3 | 0.62 |
| Tryptophan3 | 0.18 |
| Valine3 | 0.94 |
1Premix for per kilogram diet included: vitamin A, 12,000 IU; vitamin E, 24 IU; vitamin D3, 2,000 IU; vitamin K3, 2.0 mg; riboflavin, 6.0 mg; thiamine, 2.0 mg; vitamin B12, 24 ng; pyridoxine, 4 mg; pantothenic acid, 20 mg; niacin, 30 mg; biotin, 0.4 mg; folic acid, 3.6 mg; iron, 96 mg; choline chlorade, 0.4 mg; zinc, 120 mg; copper, 8.0 mg; manganese, 40 mg; selenium, 0.4 mg; iodine, 0.56 mg.
2Calculated values.
3Analyzed values.
After weaning, based on the initial BW and source litter, 192 piglets (Duroc × [Landrace × Yorkshire], average weight of 6.98 ± 0.32 kg, weaned at day 21) were selected and allotted into four dietary treatments with eight replicate pens per treatment, three barrows and three gilts per pen for a 4-wk study (phase 1: day 1 to 14; phase 2: day 15 to 28). The supplemented amount of FSE was also 100 mg/kg during nursery period. The treatments included the following: 1) CC (sows and their piglets both fed control diet); 2) CF (sows fed control diet and their piglets fed FSE diet); 3) FC (sows fed FSE diet and their piglets fed control diet); and 4) FF (sows and their piglets both fed FSE diet). The ingredients and nutrient levels in basal diet for nursery pigs was shown in Table 2. The nutrient levels met or exceeded the recommended requirement suggested in NRC (2012).
Table 2.
Ingredients and nutrient composition in basal diets of piglets (%, as-fed basis)
| Items | Phase 1 (day 1 to 14) | Phase 2 (day 15 to 28) |
|---|---|---|
| Ingredients | ||
| Corn | 60.00 | 63.00 |
| Soybean meal | 10.70 | 15.50 |
| Extruded soybean | 12.00 | 8.00 |
| Fish meal | 4.00 | 4.00 |
| Whey powder, 3.8% | 4.00 | 4.00 |
| Spray dried plasma protein | 3.00 | 4.00 |
| Soy oil | 2.86 | 2.28 |
| Dicalcium phosphate | 0.75 | 0.65 |
| Limestone | 1.02 | 0.80 |
| Salt | 0.30 | 0.30 |
| L-lysine HCl | 0.39 | 0.45 |
| DL-Methionine | 0.10 | 0.08 |
| L-Threonine | 0.10 | 0.15 |
| L-Tryptophan | 0.03 | 0.04 |
| Chromic oxide | 0.25 | 0.25 |
| Vitamin-mineral premix1 | 0.50 | 0.50 |
| Nutrient level (analyzed values) | ||
| Gross energy, MJ/kg | 14.82 | 14.60 |
| Crude protein | 19.76 | 18.36 |
| Calcium | 0.79 | 0.68 |
| Total phosphorus | 0.68 | 0.59 |
| Lysine | 1.35 | 1.23 |
| Methionine | 0.76 | 0.68 |
| Threonine | 0.79 | 0.73 |
| Tryptophan | 0.22 | 0.20 |
1Premix provided the following per kg of feed: vitamin A, 12,000 IU; vitamin E, 30 IU; vitamin D3, 2,500 IU; vitamin K3, 30 mg; riboflavin, 4 mg; vitamin B12, 12 μg; niacin, 40 mg; pantothenic acid, 15 mg; folic acid, 0.7 mg; choline chloride, 400 mg; vitamin B6, 3 mg; biotin, 0.1 mg; vitamin B1, 1.5 mg; iron, 90 mg; manganese, 40 mg; copper, 8.8 mg; zinc, 100 mg; selenium, 0.3 mg; iodine, 0.35 mg.
In the nursery room, the temperature was kept at 24 ± 2 °C, and the relative humidity was maintained at 60% to 70%. Pigs were housed in 1.5 × 1.5 m experimental pens with plastic slatted floors, duckbill drinkers, and adjustable feeders. Pigs were fed ad libitum in mash form and had free access to water. The diarrhea score was recorded daily and the diarrhea rate was calculated following the method recommended by Long et al. (2018). On day 1, 14, and 28, pigs and feed were weighed to calculated the average daily gain (ADG), average daily feed intake (ADFI), and feed efficiency (FE, ADG/ADFI).
Experimental sample collection and analysis
Within 2 h post-farrowing, four reproductive glands (first and last on both sides, 15 mL of milk per gland) were used for the collection of colostrum samples and milk samples (via infusing 10 IU oxytocin, n = 12) on day 7 and 21 of lactation, and then stored at −20 °C immediately after collection. The contents of protein, lactose, and fat in colostrum and milk samples were measured via a Milkoscan System 4000 (Foss North America, Eden Prairie, MN; AOAC, 1990).
On day 21 of lactation, the serum samples were collected from sows and suckling piglets via jugular vein puncture into vacutainer (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ). After stewing for 3 h, blood samples were centrifuged at 3,000 × g for 10 min at 4 °C to collect serum samples, and then frozen at −20 °C until analysis. These serum samples were used to measure superoxide dismutase (SOD) and total antioxidant capacity (T-AOC) via a spectrophotometer (Leng Guang SFZ1606017568, Shanghai, China) following the instructions of the kit’s manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
The grab sample technique and rectal palpation were used to collect about 1 kg clean and fresh fecal samples in each pen from day 12 to 14 during phase 1 and day 26 to 28 during phase 2, and these fecal samples were dried for 72 h (at 65 °C). About 1 kg feed samples were obtained weekly during the experiment. All feed and fecal samples were ground to pass through a 1-mm sieve and measured the dry matter (DM), ether extract, ash, crude protein, and neutral detergent fiber (NDF) according to the methods of AOAC (2012). The chromium content in feed and fecal samples was measured using an atomic absorption spectrophotometer (Z-5000; Hitachi, Tokyo, Japan). The gross energy was measured via an automatic isoperibolic oxygen bomb calorimeter (Parr 1281, Automatic Energy Analyzer; Moline, IL). The organic matter was equal to DM subtract ash. The apparent total tract digestibility (ATTD) of nutrients was calculated as following: ATTDnutrient (%) = (1− [Crdiet × Nutrientfeces]/[Crfeces × Nutrientdiet]) × 100.
After 12 h of starvation, one barrow close to average BW in each pen was used to collect about 8 mL blood samples via jugular vein puncture into vacutainer (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ) on the morning of day 14 and 28. After stewing for 3 h, blood samples were centrifuged at 3,000 × g for 10 min at 4 °C to collect serum samples, and then frozen at −20 °C until analysis. These serum samples were used to measure the SOD, T-AOC, malondialdehyde (MDA), glutathione peroxidase (GSH-Px), and tumor nuclear factor-α (TNF-α) contents via a spectrophotometer (Leng Guang SFZ1606017568, Shanghai, China) following the instructions of the kit’s manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The serum samples (day 28) were also used to measure the immunoglobulins (IgG, IgM, and IgA) via an ELISA kit (IgG, IgM, and IgA quantitation kit; Bethyl Laboratories, Inc., Texas), growth hormone (GH) by using radioimmunoassay (Sn-96513, Shanghai, China).
On day 28, one barrow (close to the average BW in each pen) was slaughtered to collect aseptic intestinal samples (duodenum, jejunum, and ileum) for testing intestinal morphology (about 5 cm fragment in the middle of each intestine, n = 8). The jejunal mucosa were carefully scraped using a sterile glass slide, and the digesta samples in the middle part of cecum and colon were collected. All the jejunal mucosa and digesta samples were placed in a 2-mL cryotube, frozen in liquid nitrogen immediately, and then stored at −80 °C until analysis.
Analysis of gut morphology
The histological samples were immediately fixed in 50 mL tubes filled with 10% neutral buffered formalin for about 48 h, and then washed, excised, dehydrated, and embedded in the paraffin wax. About five transverse sections of these histological tissues were sliced, installed on glass slides, and dyed with eosin and hematoxylin. A calibrated 10-fold eyepiece graticule was used to measure about 15 orientated villi and their adjoining crypts randomly on each slice for the calculation of the average villus height, crypt depth, and their ratio (villus height/crypt depth).
The protein extraction and analysis in jejunal mucosa
The protein extraction and analysis in jejunal mucosa (five piglets near the average BW in each group, n = 5) were carried out according to the procedure of Hu et al. (2019). Briefly, the ProteoJET Total Protein Extraction Kit (Fermentas, Hanover, MD) was used to extract protein in the mucosa of jejunum. The BCA Protein Assay Kit (Beyotime, Shanghai, China) was used to determine the content of protein. The 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis was used to separate the protein, and then the protein was transferred onto an activated polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA). The 5% Bovine Serum Albumin-Tris-Buffered Saline and Tween was used to block the membrane for 60 min and the TBST was used to wash the membrane for three times. Then, appropriate primary antibodies were used to incubate the membrane overnight (at 4 °C). The primary antibodies included rabbit anti-occludin (1: 200, Abcam) and rabbit anti-beta Actin (1: 1,000, Abcam). A goat anti-rabbit IgG secondary antibody was used to incubate the membrane for 1 h. The Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE) was used to visualize the target bands, while the Quantity One Software (BioRad Laboratories) was used to analyze the bolt. The target protein-to-β-actin protein ratio was used to express the relative abundance of occludin in jejunal mucosa.
Analysis of gut microbiota composition
The digesta samples in cecum and colon of piglets (six piglets near the average BW in each group, n = 6) were also used for the measurement of microbiota community. The DNA kit (Omega bio-tek, Norcross) was used to extract bacterial genomic DNA from digesta samples. A pair of primers (338F: ACTCCTACGGGAGGCAGCAG, 806R: GGACTACHVGGGTWTCTAAT) targeting the V3-V4 region were used to amplify the 16S rRNA of samples. The polymerase chain reaction (PCR) was done following the instruction of the KAPA HiFiHotstart PCR kit. Then, 2% agarose gel was used to extract the PCR products and the AxyPrep DNA gel extraction kit (Axygen Biosciences, California) was used to purify the PCR products. These products were combined and Illumina MiSeq platform was used to pair the terminal sequencing (2 × 250 bp). The QIIME (version 1.17) was used to demultiplex and quality filter the original fastq files. The sequences with overlapping exceeding 10 bp were assembled. The operational classification unit (OTU) (97% similarity) was categorized by using UPARSE, and the chimeric sequences were identified and removed by using UCHIME. Based on the analysis of the classification (Cole et al., 2014), the RDP classifier (at 80% confidence level) to RDP OTU database (https://rdp.cme.msu.edu/) was performed in this study.
Statistical analysis
Before weaning, the litter was used as the experimental unit and a Student’s t-test was used to analyze the pre-weaning mortality, litter size at birth, litter birth weight, and litter weight. The individual sow or suckling piglet was used as the experimental unit to analyze the milk composition and serum samples. After weaning, the pen or individual pig was used as the experimental unit, and the MIXED procedures of SAS (SAS Inst. Inc., Cary, NC) for a split-plot arrangement with sow diet as the whole plot and nursery diet as split plot were used to analyze the performance, nutrient digestibility, serum biochemical index, gut morphology, barrier function, and microbiota data. Means were separated using PDIFF option of SAS (version 9.2, 2008) (Hang et al., 2019). The differences of diarrhea score and diarrhea rate were analyzed by chi-square contingency test. Significant difference was defined as P ≤ 0.05, and a trend of difference was defined as 0.05 < P ≤ 0.10.
Results
Performance for suckling piglets and milk composition
As given in Table 3, the litter size at birth, pre-weaning mortality, litter birth weight, litter weight at day 7 and 14, and litter weaning weight were not different between the control and FSE group (Table 3). There is a numerical enhancement of litter weaning weight (60.3 vs. 65.2 kg) for FSE-supplemented sows. Moreover, on day 7 of lactation, the level of fat in milk was improved (P < 0.05) in FSE group compared with control.
Table 3.
Effects of dietary Forsythia suspensa extract supplementation on performance of suckling piglets and milk composition (n = 12)
| Items | Control | Forsythia suspensa extract | SEM | P-value |
|---|---|---|---|---|
| Average parity of sows | 3.25 | 3.50 | 4.43 | 0.79 |
| Initial BW of sows, kg | 233 | 235 | 6.81 | 0.75 |
| Initial back fat of sows, mm | 15.8 | 16.3 | 1.19 | 0.77 |
| Litter size at birth | 10.3 | 10.0 | 0.12 | 0.17 |
| Pre-weaning mortality, % | 0.25 | 0.00 | 0.12 | 0.17 |
| Litter birth weight, kg | 15.0 | 16.0 | 0.85 | 0.42 |
| Litter weight at day 7, kg | 28.0 | 28.7 | 1.37 | 0.72 |
| Litter weight at day 14, kg | 44.2 | 46.3 | 1.49 | 0.36 |
| Litter weaning weight, kg | 60.3 | 65.2 | 2.15 | 0.15 |
| Colostrum and milk composition, % | ||||
| Colostrum | ||||
| Fat | 3.67 | 4.35 | 0.59 | 0.53 |
| Protein | 16.1 | 15.3 | 1.78 | 0.86 |
| Lactose | 2.26 | 2.44 | 0.35 | 0.78 |
| Milk on day 7 | ||||
| Fat | 3.12 | 3.75 | 0.13 | 0.03 |
| Protein | 2.49 | 2.51 | 0.04 | 0.77 |
| Lactose | 2.99 | 2.99 | 0.06 | 0.98 |
| Milk at weaning | ||||
| Fat | 3.43 | 3.53 | 0.21 | 0.75 |
| Protein | 2.51 | 2.60 | 0.10 | 0.57 |
| Lactose | 2.91 | 3.02 | 0.06 | 0.30 |
Antioxidant status in serum of sows and suckling piglets
As given in Table 4, compared with control, the serum SOD and T-AOC levels in FSE-fed sows were increased (P < 0.05), while the serum T-AOC level in suckling piglets from FSE-fed sows was increased (P < 0.05), the SOD level in suckling piglets from FSE-fed sows tended to be increased (P = 0.10).
Table 4.
Effects of dietary Forsythia suspensa extract supplementation on antioxidant status in serum of sows and suckling piglets (n = 12)
| Items, U/mL | Control | Forsythia suspensa extract | SEM | P-value |
|---|---|---|---|---|
| Sows | ||||
| Superoxide dismutase | 89.25 | 109.42 | 3.92 | 0.02 |
| Total antioxidant capacity | 5.75 | 6.83 | 0.23 | 0.03 |
| Piglets | ||||
| Superoxide dismutase | 99.00 | 119.68 | 6.70 | 0.10 |
| Total antioxidant capacity | 9.46 | 13.55 | 0.59 | <0.01 |
Performance and diarrhea incidence for nursery piglets
As given in Table 5, piglets from FSE-fed sows tended to show higher ADG in phase 1 (P = 0.10) and 2 (P = 0.09), and improved (P < 0.05) ADG and FE in overall (day 1 to 28) compared with those from sows fed control diet.
Table 5.
Effects of dietary Forsythia suspensa extract supplementation to lactating sows and nursery pigs on post-weaning performance
| Sow diet | Control | FSE1 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Nursery diet | Control | FSE | Control | FSE | Sow diet | Nursery diet | Sow diet × Nursery diet | |
| BW, kg | ||||||||
| Day 1 | 6.96 | 6.98 | 6.99 | 6.98 | 0.32 | 0.96 | 1.00 | 0.96 |
| Day 14 | 9.67 | 10.13 | 10.09 | 10.04 | 0.30 | 0.57 | 0.50 | 0.42 |
| Day 28 | 14.39 | 15.19 | 15.52 | 15.29 | 0.45 | 0.18 | 0.53 | 0.26 |
| Day 1 to 14 | ||||||||
| ADG, g | 195 | 211 | 222 | 217 | 9.96 | 0.10 | 0.58 | 0.32 |
| ADFI, g | 324 | 331 | 348 | 335 | 16.5 | 0.40 | 0.83 | 0.56 |
| Feed efficiency | 0.61 | 0.64 | 0.64 | 0.65 | 0.02 | 0.31 | 0.41 | 0.66 |
| Diarrhea score | 3.40 | 3.21 | 3.12 | 3.10 | 0.05 | <0.01 | 0.03 | 0.09 |
| Diarrhea rate | 11.7 | 4.4 | 2.9 | 1.9 | 2.09 | <0.01 | 0.06 | 0.14 |
| Day 15 to 28 | ||||||||
| ADG, g | 363 | 389 | 418 | 404 | 19.5 | 0.09 | 0.75 | 0.31 |
| ADFI, g | 650 | 672 | 702 | 662 | 23.9 | 0.38 | 0.70 | 0.20 |
| Feed efficiency | 0.56 | 0.58 | 0.60 | 0.61 | 0.03 | 0.14 | 0.44 | 0.91 |
| Diarrhea score | 3.28 | 3.10 | 3.08 | 3.03 | 0.03 | < 0.01 | < 0.01 | 0.06 |
| Diarrhea rate | 5.6 | 3.2 | 2.7 | 1.5 | 1.16 | 0.06 | 0.14 | 0.61 |
| Day 1 to 28 | ||||||||
| ADG, g | 276 | 297 | 316 | 307 | 10.5 | 0.03 | 0.58 | 0.17 |
| ADFI, g | 487 | 502 | 525 | 499 | 17.0 | 0.31 | 0.71 | 0.24 |
| Feed efficiency | 0.57 | 0.59 | 0.60 | 0.62 | 0.01 | 0.05 | 0.17 | 0.67 |
| Diarrhea score | 3.33a | 3.15b | 3.10b | 3.06b | 0.03 | <0.01 | <0.01 | 0.03 |
| Diarrhea rate | 8.7 | 3.8 | 2.8 | 1.7 | 1.58 | 0.02 | 0.07 | 0.24 |
1FSE, Forsythia suspensa extract.
a,bDifferent superscripts within a row mean significant difference (P < 0.05).
In phase 1, the diarrhea score and diarrhea rate were lower (P < 0.05) in piglets from sows supplemented with FSE compared with those from sows fed control diet. Dietary supplementation with FSE during nursery had lower (P < 0.05) diarrhea score and tended to have reduced (P = 0.06) diarrhea rate compared with those fed control diet during nursery. In phase 2, piglets from FSE-fed sows had lower (P < 0.05) diarrhea score and tended to show lower (P = 0.06) diarrhea rate compared with those from sows fed control diet. Piglets supplemented with FSE during nursery had lower (P < 0.05) diarrhea score compared with those fed control diet during nursery. In overall, piglets from sows supplemented with FSE have lower (P < 0.05) diarrhea score and diarrhea rate compared with those from sows fed control diet. Piglets supplemented with FSE during nursery had lower (P < 0.05) diarrhea score and tended to show reduced (P = 0.07) diarrhea rate compared with those fed control diet during nursery. Moreover, there was an interaction between FSE-fed in sow diet and FSE-fed in nursery diet on reducing (P < 0.05) diarrhea score. Compared with piglets from CC group, piglets from CF, FF, and FC groups had lower (P < 0.05) diarrhea score.
The ATTD of nutrients
As given in Table 6, in phase 1, piglets from FSE-fed sows had enhanced (P < 0.05) ATTD of NDF compared with those from sows fed control diet.
Table 6.
Effects of dietary Forsythia suspensa extract supplementation to lactating sows and nursery pigs on post-weaning apparent total tract digestibility of nutrients (%) (n = 8)
| Sow diet | Control | FSE1 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Nursery diet | Control | FSE | Control | FSE | Sow diet | Nursery diet | Sow diet × Nursery diet | |
| Day 14 | ||||||||
| DM | 81.21 | 81.12 | 79.94 | 78.97 | 1.25 | 0.19 | 0.68 | 0.73 |
| Gross energy | 58.58 | 61.98 | 57.87 | 53.97 | 3.11 | 0.18 | 0.94 | 0.26 |
| Organic matter | 82.17 | 82.42 | 82.34 | 81.78 | 0.64 | 0.72 | 0.82 | 0.54 |
| Ether extract | 80.91 | 81.11 | 80.53 | 79.86 | 0.55 | 0.15 | 0.67 | 0.44 |
| Neutral detergent fiber | 81.11 | 81.34 | 84.27 | 83.70 | 0.64 | < 0.01 | 0.79 | 0.54 |
| Day 28 | ||||||||
| DM | 61.45 | 67.45 | 63.96 | 63.38 | 1.88 | 0.69 | 0.17 | 0.10 |
| Gross energy | 79.00 | 79.73 | 78.57 | 77.72 | 1.45 | 0.43 | 0.97 | 0.60 |
| Organic matter | 82.93 | 83.14 | 83.44 | 81.12 | 1.30 | 0.58 | 0.44 | 0.36 |
| Ether extract | 60.78 | 68.51 | 61.72 | 60.36 | 3.65 | 0.36 | 0.41 | 0.25 |
| NDF | 59.48 | 60.15 | 59.60 | 60.39 | 1.71 | 0.91 | 0.67 | 0.97 |
1FSE, Forsythia suspensa extract.
a,bDifferent superscripts within a row mean significant difference (P < 0.05).
Antioxidant status and TNF-α
As given in Table 7, on day 14, piglets from FSE-fed sows have enhanced (P < 0.05) content of GSH-Px in serum compared with those from sows fed control diet. There was an interaction between FSE-fed in sow diet and FSE-fed in nursery diet on increasing (P < 0.05) the concentrations of SOD, GSH-Px, and catalase (CAT) in serum. Compared with piglets from CC group, piglets from CF and FC groups had greater (P < 0.05) SOD and CAT levels in serum, while piglets from FC group had increased (P < 0.05) GSH-Px level in serum.
Table 7.
Effects of dietary Forsythia suspensa extract supplementation to lactating sows and nursery pigs on post-weaning antioxidant status and immune function in serum (n = 8)
| Sow diet | Control | FSE1 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Nursery diet | Control | FSE | Control | FSE | Sow diet | Nursery diet | Sow diet × Nursery diet | |
| Day 14 | ||||||||
| SOD, U/mL | 139b | 143a | 141a | 136b | 2.16 | 0.23 | 0.77 | 0.04 |
| T-AOC, U/mL | 4.71 | 5.85 | 6.21 | 5.94 | 0.52 | 0.14 | 0.40 | 0.19 |
| GSH-Px, U/mL | 700b | 732b | 873a | 741b | 38.38 | 0.03 | 0.21 | 0.04 |
| CAT, U/mL | 5.47b | 6.55a | 6.21a | 5.49b | 0.37 | 0.68 | 0.64 | 0.02 |
| MDA, nmol/mL | 4.01 | 3.58 | 3.80 | 4.00 | 0.36 | 0.72 | 0.80 | 0.35 |
| TNF-α, pg/mL | 67.72 | 64.35 | 64.89 | 63.11 | 3.82 | 0.60 | 0.51 | 0.84 |
| Day 28 | ||||||||
| SOD, U/mL | 112 | 120 | 134 | 139 | 1.68 | < 0.01 | 0.01 | 0.51 |
| T-AOC, U/mL | 10.45c | 11.69bc | 14.31a | 12.70b | 0.29 | < 0.01 | 0.66 | < 0.01 |
| GSH-Px, U/mL | 577c | 688b | 707a | 618b | 20.35 | 0.32 | 0.69 | < 0.01 |
| CAT, U/mL | 5.28 | 6.96 | 5.65 | 5.97 | 0.30 | 0.48 | 0.04 | 0.14 |
| MDA, nmol/mL | 5.92 | 3.99 | 4.04 | 3.55 | 0.30 | 0.02 | 0.01 | 0.11 |
| TNF-α, pg/mL | 73.79a | 67.24ab | 57.40b | 68.15ab | 2.17 | < 0.02 | 0.51 | 0.03 |
| IgG, g/L | 7.98 | 9.62 | 9.86 | 11.18 | 0.30 | < 0.01 | < 0.01 | 0.72 |
| IgA, g/L | 0.73b | 0.80ab | 0.83a | 0.70b | 0.03 | 0.96 | 0.57 | 0.04 |
| IgM, g/L | 0.73 | 0.78 | 0.74 | 0.70 | 0.03 | 0.37 | 0.88 | 0.32 |
| Gross hormone, ng/mL | 3.65 | 3.56 | 5.40 | 4.88 | 0.28 | < 0.01 | 0.46 | 0.60 |
1FSE, Forsythia suspensa extract.
a–cDifferent superscripts within a row mean significant difference (P < 0.05).
On day 28, piglets from FSE-fed sows showed higher (P < 0.05) content of SOD and T-AOC, and lower (P < 0.05) MDA and TNF-α levels in serum compared with those from sows fed control diet. Dietary supplementation with FSE during nursery had increased (P < 0.05) concentrations of SOD and CAT, and decreased (P < 0.05) MDA level in serum compared with those fed control diet during nursery. There was an interaction between FSE-fed in sow diet and FSE-fed in nursery diet on the concentrations of T-AOC, GSH-Px, and TNF-α in serum (P < 0.05). Compared with piglets from CC group, piglets from FF and FC groups had greater (P < 0.05) T-AOC and GSH-Px levels in serum, while piglets from FC group had lower (P < 0.05) TNF-α level in serum.
Serum GH and immunoglobulins
As given in Table 7, on day 28, piglets from FSE-fed sows had higher (P < 0.05) content of IgG and GH in serum compared with those from sows fed control diet. Dietary supplementation with FSE during nursery had increased (P < 0.05) concentrations of IgG in serum compared with those fed control diet during nursery. There was an interaction between FSE-fed in sow diet and FSE-fed in nursery diet on increasing (P < 0.05) the IgA level in serum. Piglets from FC group had higher (P < 0.05) IgA level in serum compared with piglets from CC or FF group.
Intestinal morphology
As given in Table 8, compared with piglets from sows fed control diet, piglets from FSE-fed sows had higher (P < 0.05) villus height in ileum and villus height to crypt depth ratio in jejunum, lower (P < 0.05) crypt depth in ileum, as well as a tendency of greater (P = 0.07) villus height in duodenum. There was an interaction between FSE-fed in sow diet and FSE-fed in nursery diet on improving (P < 0.05) villus height to crypt depth ratio in jejunum, piglets from FC group had higher (P < 0.05) villus height to crypt depth ratio in jejunum compared with piglets from CC group.
Table 8.
Effects of dietary Forsythia suspensa extract supplementation to lactating sows and nursery pigs on post-weaning intestinal morphology (n = 8)
| Sow diet | Control | FSE1 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Nursery diet | Control | FSE | Control | FSE | Sow diet | Nursery diet | Sow diet × Nursery diet | |
| Duodenum | ||||||||
| Villus height, μm | 435 | 435 | 506 | 510 | 25.02 | 0.07 | 0.95 | 0.95 |
| Crypt depth, μm | 321 | 297 | 353 | 325 | 16.43 | 0.23 | 0.30 | 0.93 |
| Villus height/Crypt depth | 1.37 | 1.52 | 1.49 | 1.61 | 0.06 | 0.25 | 0.15 | 0.89 |
| Jejunum | ||||||||
| Villus height, μm | 393 | 446 | 518 | 461 | 25.33 | 0.08 | 0.96 | 0.16 |
| Crypt depth, μm | 247 | 256 | 272 | 263 | 17.69 | 0.55 | 1.00 | 0.72 |
| Villus height/Crypt depth | 1.62b | 1.77ab | 1.95a | 1.79ab | 0.04 | 0.01 | 1.00 | 0.02 |
| Ileum | ||||||||
| Villus height, μm | 379 | 344 | 431 | 420 | 11.38 | < 0.01 | 0.19 | 0.48 |
| Crypt depth, μm | 294 | 191 | 233 | 229 | 22.10 | < 0.01 | 0.12 | 0.15 |
| Villus height/Crypt depth | 1.49 | 1.87 | 1.89 | 1.92 | 0.14 | 0.30 | 0.34 | 0.41 |
1FSE, Forsythia suspensa extract.
a,bDifferent superscripts within a row mean significant difference (P < 0.05).
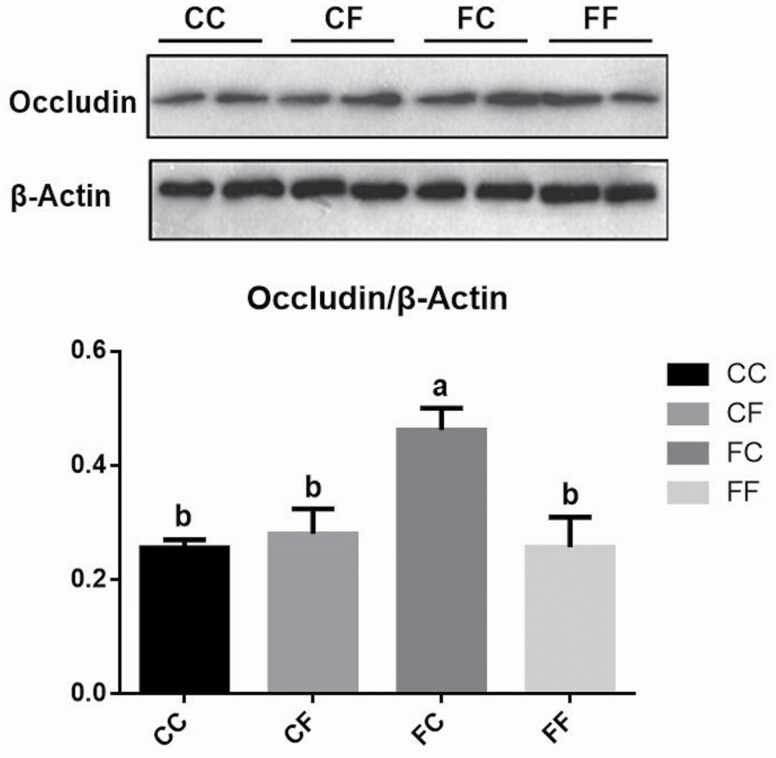
Occludin protein expression in jenual mucosa
According to Figure 1, piglets from FC group had increased (P < 0.05) protein expression of occludin in jejunal mucosa compared with other groups.
Figure 1.
Effects of dietary FSE supplementation to lactating sows and nursery pigs on post-weaning barrier function in jejunal mocusa (n = 5). Note: CC: sows and their piglets both fed control diet; FC: sows fed FSE diet and their piglets fed control diet; CF: sows fed control diet and their piglets fed FSE diet; FF: sows and their piglets both fed FSE diet. a,bDifferent superscripts mean significant difference (P < 0.05).
Gut microbiota community
According to Table 9, the main microbiota community of CC, FC, CF, and FF were Firmicutes, Bacteroidetes, Protebacteria, and Actinobacteria on phylum level, and Lactobacillus, Streptoccus, Prevotella_9, and Prevotellaceae_NK3B31_group on genus level. Compared with piglets from sows fed control diet, piglets from FSE-fed sows had higher (P < 0.05) relative abundance of Lactobacillus on genus level in cecum and colon, increased (P < 0.05) relative abundance of Firmicutes on phylum level in colon, and decreased (P < 0.05) relative abundance of Prevotellaceae_NK3B31_group on genus level in cecum. There was an interaction between FSE-fed in sow diet and FSE-fed in nursery diet on improving (P < 0.05) Lactobacillus on genus level in colon; piglets from FC group had higher (P < 0.05) Lactobacillus on genus level in colon compared with piglets from CC group.
Table 9.
The relative abundance of microbiota on phylum and genus level in cecum and colon of nursery pigs (%, n = 6)
| Sow diet | Control | FSE1 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Nursery diet | Control | FSE | Control | FSE | Sow diet | Nursery diet | Sow diet × Nursery diet | |
| Cecum | ||||||||
| Phylum level | ||||||||
| Firmicutes | 68.26 | 83.81 | 88.08 | 93.06 | 7.63 | 0.08 | 0.20 | 0.50 |
| Bacteroidetes | 28.40 | 12.05 | 7.36 | 3.82 | 7.71 | 0.08 | 0.22 | 0.42 |
| Proteobacteria | 1.77 | 1.73 | 3.87 | 1.88 | 1.10 | 0.33 | 0.38 | 0.40 |
| Genus level | ||||||||
| Lactobacillus | 10.03 | 17.67 | 44.27 | 31.50 | 7.05 | < 0.01 | 0.72 | 0.17 |
| Streptococcus | 8.22 | 9.74 | 12.96 | 13.80 | 4.24 | 0.32 | 0.79 | 0.94 |
| Prevotella_9 | 7.33 | 5.66 | 3.84 | 2.35 | 2.82 | 0.25 | 0.59 | 0.98 |
| Prevotellaceae_NK3B31_group | 5.65 | 3.83 | 0.79 | 0.35 | 1.30 | < 0.01 | 0.40 | 0.61 |
| Colon | ||||||||
| Phylum level | ||||||||
| Firmicutes | 61.67 | 74.77 | 86.83 | 76.80 | 5.85 | 0.04 | 0.80 | 0.07 |
| Bacteroidetes | 34.26 | 20.03 | 10.05 | 19.94 | 5.72 | 0.06 | 0.71 | 0.06 |
| Proteobacteria | 1.42 | 2.30 | 1.66 | 1.74 | 0.66 | 0.81 | 0.48 | 0.56 |
| Genus level | ||||||||
| Lactobacillus | 5.19b | 17.01ab | 30.19a | 16.11ab | 4.28 | 0.02 | 0.80 | 0.01 |
| Streptococcus | 5.25 | 8.99 | 13.38 | 9.70 | 2.73 | 0.13 | 0.99 | 0.20 |
| Prevotella_9 | 9.11 | 9.47 | 3.59 | 7.21 | 3.18 | 0.24 | 0.54 | 0.62 |
| Prevotellaceae_NK3B31_group | 6.07 | 2.82 | 1.41 | 3.37 | 1.13 | 0.09 | 0.58 | 0.06 |
1FSE, Forsythia suspensa extract.
a,bDifferent superscripts within a row mean significant difference (P < 0.05).
Discussion
Weaning stress is an inescapable process for piglets, which might result in a reduction of performance in piglets after weaning (Campbell et al., 2013), while adding feed additives in diet of nursery pigs could help alleviate weaning stress (Vondruskova et al., 2010). However, most studies only focus on using feed additives in the diet of nursery pigs to modulate their performance, while using feed additives during lactation period in sow diet to alleviate weaning stress is often not taken into consideration. Recently, Hang et al. (2019) reported that diet supplementation with live yeast in the diet of sows and the diet of nursery pigs could enhance post-weaning ADG and alleviate weaning stress effectively. Therefore, this experiment focusing on supplying FSE in feed of both nursery pigs and lactating sows, which aimed to evaluate the maternal FSE supplementation on performance of nursery pigs and seek a better way to solve weaning stress problem.
A reduction of feed intake and poor performance were often occurred in pigs after weaning (Kojima et al., 2007). In this study, piglets from FSE-fed sows had improved ADG and FE in overall period after weaning compared with those from sows fed control diet, which indicated that maternal FSE supplementation in sows could effectively improve the performance and alleviate weaning stress in nursery pigs. Our previous studies have demonstrated that dietary FSE supplementation could effectively increased ADG or FE in nursery pigs via improving nutrient utilization, antioxidant capacity, and immunoglobulins (Zhao et al., 2017a; Long et al., 2019). Therefore, we suspected that the current finding was possibly due to the beneficial maternal effects of FSE (especially the forsythiaside A) on improving antioxidant status and immunoglobulins in sows and their offspring (Long et al., 2021). Moreover, the concentration of GH in piglets from FSE-fed sows could explain the improved performance in nursery pigs since the GH could enhance the growth rate in animals (Long et al., 2018). The gut microbiota composition could also be improved by FSE since FSE could reduce the content of Escherichia coli and increase the content of Lactobacillus (Han et al., 2012), which indicated that maternal FSE supplementation might improve the gut health in their offspring and thus enhance post-weaning performance.
In this study, we found that the performance in FSE supplemented in lactation period was better than nursery period. One of the possible reasons might be that FSE could increase the level of milk fat on day 7 of lactation; this might explain the enhanced health status in offsprings of FSE-fed sows since milk fat supply energy to suckling piglets. Long et al. (2021) has reported that maternal FSE supplementation could increase the antioxidant capacity in colostrum and serum of sows and newborn piglets. In this study, FSE increased the serum SOD and T-AOC levels in sows and suckling piglets, which revealed that the enhanced antioxidant capacity of FSE-fed sows may pass from sows to piglets.
Diarrhea is one of the most difficult problems for nursery pigs, which always influences the growth performance of piglets and results in the death of piglets. Martinez-Puig et al. (2007) reported that nucleotides from yeast might prevent post-weaning diarrhea in piglets, indicating that maternal functional nutrients supplementation could alleviate post-weaning diarrhea. In this study, piglets from CF, FF, and FC groups had lower diarrhea score compared with CC (ranged from 3.06 to 3.33). Although diarrhea score in this study varied in small range, it revealed that FSE may play a role in reducing the post-weaning diarrhea in nursery pigs (Zhao et al., 2012; Long et al., 2018).
The diarrhea rate in this study indicated that the addition of FSE to the maternal and offspring both had a positive influence on the reduction of post-weaning diarrhea. After weaning, the villous atrophy and crypt hyperplasia showed in the small intestine of piglets might cause digestive disorders and diarrhea in piglets (Pluske et al., 1997). Escherichia coli could lead to diarrhea (Fairbrother et al., 2005), while FSE could decrease the harmful bacteria in large intestine of piglets, especially E. coli (Long et al., 2019). Therefore, we speculated that the effect of FSE on reducing diarrhea rate might be related to its role of increasing antioxidant activities, immunity, and modulating intestinal microbiota community in nursery pigs. It is proved that offsprings could receive maternal microbes (Funkhouser and Bordenstein, 2013) and immunoglobulins (Peri and Rothberg, 1986) through placenta, colostrums, and milk. As hypothesized, the lower diarrhea rate and diarrhea score, observed in piglets from FSE-fed sows, may be also due to that piglets obtain more intestinal beneficial bacteria and antibodies from sows.
Stress might also lead to impairment of gut morphology and barrier function (Liu et al., 2014). For the nursery pigs, the development of gut morphology is important for their utilization for nutrients (including protein, lactose, and lipid) from diet. We also observed that dietary FSE supplementation in sow and nursery pigs both showed the increased villus height to crypt depth ratios in jejunum, while the villus height and crypt depth in ileum also increased significantly when FSE only supplemented to nursery diet. Piglets from FC group had higher villus height to crypt depth ratio in jejunum compared with piglets from CC group. This finding was mainly due to that FSE could modulate intestinal permeability, alleviate intestinal injure in piglets (Zhao et al., 2017a), and repair the epidermis damage caused by antigen protein and pathogen in feed (Han et al., 2012). In this study, maternal FSE supplementation also showed a positive trend on improving ATTD of nutrition in nursery pigs, especially the ATTD of NDF, which could be explained by the improvement of intestinal morphology and nutrient absorption ability caused by FSE (Han et al., 2012). Chen et al. (2020) also reported that Tong-fu-li-fei (containing FSE) could improve barrier function via up-regulating zona occludens 1/occludin/claudin-1 expression. In this study, the protein expression of occludin in jejunal mocusa was increased in piglets from FSE-fed sows (FC group), which was beneficial for the intestinal development and health status of nursery pigs. The epithelial barrier function was important for nutrient absorption, while the occludin protein was one of the main conjunction proteins (Feldman et al., 2005), thus the improved protein expression of occludin in jejunal mucosa might also be one of the main reasons for the improved ATTD of nutrition, ADG, and FE in nursery pigs.
Weaning could initiate the oxidative stress and apoptosis by activating mitogen-activated protein kinase pathways (Luo et al., 2016), which increased the levels of free radicals, H2O2, and MDA, while decreased the inhibitory hydroxyl ability and activities of antioxidant enzymes, such as GSH-Px and SOD (Yuan et al., 2007; Luo et al., 2016). A cumulative production of reactive oxygen species (ROS) could also result in oxidative stress (Valko et al., 2006). The current study showed pigs from CF and FC groups showed higher SOD and CAT contents than those from CC group on day 14, which indicated FSE supplemented in lactation or nursery period was efficient to improve antioxidant capacity of pigs in the first 2 wk after weaning. However, pigs from FF group only showed increased antioxidant capacity on day 28, which might be due to the accumulative effect of FSE for pigs (Long et al., 2019). Moreover, supplementation of FSE during lactation phase and nursery phase both showed increased the concentrations of antioxidant enzymes (SOD, GSH-Px, CAT, or T-AOC), and decreased level of MDA on day 28, indicating the beneficial effect of FSE on enhancing antioxidant capacity in sows and their offprings in phase 2. Piglets from FF and FC groups had greater T-AOC and GSH-Px levels in serum on day 28 compared with piglets from CC group. The mechanism of this finding might be that Forsythiaside in FSE could activate nuclear factor E2-related factor 2 (Nrf2) signaling pathway, increase the expression of antioxidant enzymes (SOD and CAT), and thus alleviate weaning stress (Yin et al., 2013; Huang et al., 2015; Pan et al., 2015; Zhao et al., 2017a). Effective antioxidant activities of Forsythiaside may be related to the presence of aromatic hydroxy function (Piao et al., 2008) and scavenging ability of free radicals (Pan et al., 2018b). It was demonstrated that dietary Forsythiaside was beneficial to prevent ROS generation and subsequent H2O2-induced oxidative stress and apoptosis activation (Huang et al., 2015). Moreover, the BW loss caused by oxidative stress could also be attenuated by FSE (Pan et al., 2018a, 2018b), which indicated that the enhanced antioxidant capacity is beneficial for the improvement of ADG of nursery pigs in the current study.
The results of the study also showed the increasing levels of IgA, IgG, and TNF-α in both sows and piglets when FSE was provided, which indicated that the immune function in nursery pigs was improved by maternal FSE supplementation (Long et al., 2021). Zhang et al. (2013) and Zeng et al. (2014) already demonstrated that FSE could increase the immunoglobulins in broilers, while the IgA and IgG were beneficial for the ADG and health status. Piglets from FC group had higher IgA level and lower TNF-α level in serum compared with CC on day 28. The reason for the decreased TNF-α in both sows and piglets when FSE was provided might be due to that Forsythiaside in FSE could prevent inflammatory cell death (Huang et al., 2015) and liver injury in nursery pigs (Wang et al., 2016). The mechanism of ant-inflammatory of Forsythiaside might be involved in the inhibition of NF-κB signaling pathway (Wang et al., 2016), and thus decrease the IL-1β, TNF-α, and cyclooxygenase-2 levels (Cheng et al., 2014).
Weaning results in transient and long-lasting changes in intestinal physiology and quickly shift in gut microbiota of piglets (Boudry et al., 2004; Chen et al., 2017). Therefore, nursery piglets are highly vulnerable to diarrhea that caused by pathogenic bacteria (Pluske, 2013). In the distal intestine of piglets, the prevailing microbiota were Bacteroidetes and Firmicutes, including Prevotella_9, Prevotellaceae_NK3B31_group, and so on (Liu et al., 2018). Xu et al. (2020) reported that dietary supplementation with compound probiotics and berberine could increase the relative abundance of Firmicutes, and decrease the relative abundance of Prevotellaceae_NK3B31_group in large intestine of piglets, which was beneficial for improving health and immunity in piglets. In this study, compared with piglets from sows fed control diet, piglets from FSE-fed sows also had increased relative abundance of Firmicutes on phylum level in colon, and decreased relative abundance of Prevotellaceae_NK3B31_group on genus level in cecum, which was beneficial for the health and immunity for nursery pigs. The current study also showed compared with piglets from sows fed control diet, piglets from FSE-fed sows had higher relative abundance of Lactobacillus on genus level in cecum and colon, especially piglets from FC group had highest relative abundance of Lactobacillus on genus level compared with other groups. The intestinal beneficial microbiota is also crucial to the health status via providing metabolic, immunologic, and protective functions (Yin et al., 2018). The Lactobacillus is considered as a beneficial microbe in modulating gut health by improving intestinal barrier function and immunity (Yang et al., 2018), thus the improved Lactobacillus on genus level helped explain the improved intestinal barrier function, immunity, and anti-inflammatory function in nursery pigs, reflected by the improved immunoglobulins and decreased TNF-α in the current study. One of the reasons for the present finding might be that FSE could improve gut health via decreasing harmful microbiota (such as E. coli) and improving beneficial microbiota community (such as Lactobacillus) in the large intestine of sows and suckling piglets (Hao et al., 2010; Han et al., 2012; Jiao et al., 2012; Zhang et al., 2013), which might be beneficial for the microbial homeostasis after weaning in nursery pigs. The current findings showed that the effect of FSE supplemented during lactation phase seemed more efficient than the nursery phase on benefiting gut microbiota community in nursery pigs, while the mechanism still needs to be investigated.
In conclusion, in terms of the performance, intestinal morphology, and microbiota community, dietary FSE supplementation during lactation phase seemed more efficient to alleviate weaning stress than the nursery phase. However, in terms of the antioxidant status and immunoglobulins, FSE supplemented in both phases were efficient for nursery pigs. Further researches are needed to be done to confirm the mechanism of this discovery.
Acknowledgments
This research is supported by the Beijing Municipal Natural Science Foundation (6202019) and National Natural Science Foundation of China (31772612).
Glossary
Abbreviations
- ADG
average daily gain
- ATTD
apparent total tract digestibility
- BW
body weight
- DM
dry matter
- FE
feed efficiency
- GH
growth hormone
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- NDF
neutral detergent fiber
- OTU
operational classification unit
- ROS
reactive oxygen species
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Literature Cited
- AOAC . 1990. Official method of analysis. 15th ed. Washington (DC): Off. Assoc. Anal. Chem. [Google Scholar]
- AOAC . 2012. Official methods of analysis. 19th ed. Arlington (VA): Off. Assoc. Anal. Chem. [Google Scholar]
- Boudry, G., Péron V., Le Huërou-Luron I., Lallès J. P., and Sève B.. . 2004. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J. Nutr. 134:2256–2262. doi: 10.1093/jn/134.9.2256 [DOI] [PubMed] [Google Scholar]
- Campbell, J. M., Crenshaw J. D., and Polo J.. . 2013. The biological stress of early nursery piglets. J. Anim. Sci. Biotechno. 4:19. doi: 10.1186/2049-1891-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L., Li L., Han Y., Lv B., Zou S., and Yu Q.. . 2020. Tong-fu-li-fei decoction exerts a protective effect on intestinal barrier of sepsis in rats through upregulating ZO-1/occludin/claudin-1 expression. J. Pharmacol. Sci. 143:89–96. doi: 10.1016/j.jphs.2020.02.009 [DOI] [PubMed] [Google Scholar]
- Chen, L., Xu Y., Chen X., Fang C., Zhao L., and Chen F.. . 2017. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 8:1688. doi: 10.3389/fmicb.2017.01688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, G., Zhao Y., Li H., Wu Y., Li X. X., Han Q., Dai C. S., and Li Y. H.. . 2014. Forsythiaside attenuates lipopolysaccharide-induced inflammatory responses in the bursa of Fabricius of chickens by downregulating the NF-κB signaling pathway. Exp. Ther. Med. 7:179–184. doi: 10.3892/etm.2013.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, J. R., Wang Q., Fish J. A., Chai B., McGarrell D. M., Sun Y., Brown C. T., Porras-Alfaro A., Kuske C. R., and Tiedje J. M.. . 2014. Ribosomal database project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 42:633–642. doi: 10.1093/nar/gkt1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbrother, J. M., Nadeau E., and Gyles C. L.. . 2005. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 6:17–39. doi: 10.1079/ahr2005105 [DOI] [PubMed] [Google Scholar]
- Feldman, G. J., Mullin J. M., and Ryan M. P.. . 2005. Occludin: structure, function and regulation. Adv. Drug Deliv. Rev. 57:883–917. doi: 10.1016/j.addr.2005.01.009 [DOI] [PubMed] [Google Scholar]
- Funkhouser, L. J., and Bordenstein S. R.. . 2013. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 11:e1001631. doi: 10.1371/journal.pbio.1001631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, X., Piao X. S., Zhang H. Y., Li P. F., Yi J. Q., Zhang Q., and Li P.. . 2012. Forsythia suspensa extract has the potential to substitute antibiotic in broiler chicken. Asian-Australas. J. Anim. Sci. 25:569–576. doi: 10.5713/ajas.2011.11425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang, L., Pete W., Olayiwola A., and Ajuwon K. M.. . 2019. Effect of live yeast supplementation to gestating sows and nursery piglets on postweaning growth performance and nutrient digestibility. J. Anim. Sci. 97:2534–2540. doi: 10.1093/jas/skz150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, Y., Li D. F., Piao X. L., and Piao X. S.. . 2010. Forsythia suspensa extract alleviates hypersensitivity induced by soybean β-conglycinin in nursery piglets. J. Ethnopharmacol. 128: 412–418. doi: 10.1016/j.jep.2010.01.035 [DOI] [PubMed] [Google Scholar]
- Hu, P., Zhao F., Zhu W., and Wang J.. . 2019. Effects of early-life lactoferrin intervention on growth performance, small intestinal function and gut microbiota in suckling piglets. Food Funct. 10:5361–5373. doi: 10.1039/c9fo00676a [DOI] [PubMed] [Google Scholar]
- Huang, C., Lin Y., Su H., and Ye D.. . 2015. Forsythiaside protects against hydrogen peroxide-induced oxidative stress and apoptosis in PC12 cell. Neurochem. Res. 40:27–35. doi: 10.1007/s11064-014-1461-5 [DOI] [PubMed] [Google Scholar]
- Jiao, J., Fu Y. J., Zu Y. G., Luo M., Wang Y., Zhang L., and Li J.. . 2012. Enzyme-assisted microwave hydro-distillation essential oil from Fructus forsythia, chemical constituents, and its antimicrobial and antioxidant activities. Food Chem. 134:235–243. doi: 10.1016/j.foodchem.2012.02.114 [DOI] [Google Scholar]
- Kim, S. W., Weaver A. C., Shen Y. B., and Zhao Y.. . 2013. Improving efficiency of sow productivity: nutrition and health. J. Anim. Sci. Biotechnol. 4:26. doi: 10.1186/2049-1891-4-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima, C. J., Carroll J. A., Matteri R. L., Touchette K. J., and Allee G. L.. . 2007. Effects of weaning and weaning weight on neuroendocrine regulators of feed intake in pigs. J. Anim. Sci. 85:2133–2139. doi: 10.2527/jas.2006-740 [DOI] [PubMed] [Google Scholar]
- Lallès, J. P., Boudry G., Favier C., Floc’h N. L., Luron I., Montagne L., Oswald I. P., Pié S., Piel C., and Sève B.. . 2004. Gut function and dysfunction in young pigs: physiology. Anim. Res. 53:301–316. doi: 10.1051/animres:2004018 [DOI] [Google Scholar]
- Leonard, S. G., Sweeney T., Bahar B., Lynch B. P., and O’Doherty J. V.. . 2010. Effect of maternal fish oil and seaweed extract supplementation on colostrum and milk composition, humoral immune response, and performance of suckled piglets. J. Anim. Sci. 88:2988–2997. doi: 10.2527/jas.2009-2764 [DOI] [PubMed] [Google Scholar]
- Leonard, S. G., Sweeney T., Bahar B., Lynch B. P., and O’Doherty J. V.. . 2011. Effects of dietary seaweed extract supplementation in sows and post-nursery pigs on performance, intestinal morphology, intestinal microflora and immune status. Brit. J. Nutr. 106: 688–699. doi: 10.1017/S0007114511000997 [DOI] [PubMed] [Google Scholar]
- Leonard, S. G., Sweeney T., Bahar B., and O’Doherty J. V.. . 2012. Effect of maternal seaweed extract supplementation on suckling piglet growth, humoral immunity, selected microflora, and immune response after an ex vivo lipopolysaccharide challenge. J. Anim. Sci. 90:505–514. doi: 10.2527/jas.2010-3243 [DOI] [PubMed] [Google Scholar]
- Liu, B., Wang W., Zhu X., Sun X., Xiao J., Li J. D., Cui Y., Wang C., and Shi Y.. . 2018. Response of gut microbiota to dietary fiber and metabolic interaction with SCFAs in piglets. Front. Microbiol. 28:2344. doi: 10.3389/fmicb.2018.02344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., Zhang J., Zhang S., Yang F., Thacker P. A., Zhang G., Qiao S., and Ma X.. . 2014. Oral administration of Lactobacillus fermentum I5007 favors intestinal development and alters the intestinal microbiota in formula-fed piglets. J. Agric. Food Chem. 62:860–866. doi: 10.1021/jf403288r [DOI] [PubMed] [Google Scholar]
- Long, S. F., He T. F., Wu D., Yang M., and Piao X. S.. . 2020. Forsythia suspensa extract enhances performance via the improvement of nutrient digestibility, antioxidant status, anti-inflammatory function, and gut morphology in broilers. Poult. Sci. 99:4217–4226. doi: 10.1016/j.psj.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, S. F., Liu L., Liu S. J., Mahfuz S., and Piao X. S.. . 2019. Effects of Forsythia suspense extract as an antibiotics substitute on growth performance, nutrient digestibility, serum antioxidant capacity, fecal Escherichia coli concentration and intestinal morphology of nursery piglets. Animals. 9:729. doi: 10.3390/ani9100729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, S. F., Xu Y. T., Pan L., Wang Q. Q., Wang C. L., Wu J. Y., Wu Y. Y., Han Y. M., Yun C. H., and Piao X. S.. . 2018. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for nursery piglets. Anim. Feed Sci. Technol. 235: 23–32. doi: 10.1016/j.anifeedsci.2017.08.018 [DOI] [Google Scholar]
- Long, S. F., Wu D., He T. F., and Piao X. S.. . 2021. Dietary supplementation with Forsythia suspensa extract during late gestation improves reproductive performance, colostrum composition, antioxidant status, immunoglobulin, and inflammatory cytokines in sows and newborn piglets. Anim. Feed Sci. Technol. 271:114700. doi: 10.1016/j.anifeedsci.2020.114700 [DOI] [Google Scholar]
- Lu, T., Piao X. L., Zhang Q., Wang D., Piao X. S., and Kim S. W.. . 2010. Protective effects of Forsythia suspensa extract against oxidative stress induced by diquat in rats. Food Chem. Toxicol. 48:764–770. doi: 10.1016/j.fct.2009.12.018 [DOI] [PubMed] [Google Scholar]
- Luo, Z., Zhu W., Guo Q., Luo W., Zhang J., Xu W., and Xu J.. . 2016. Weaning induced hepatic oxidative stress, apoptosis, and aminotransferases through MAPK signaling pathways in piglets. Oxid. Med. Cell. Longev. 2016:4768541. doi: 10.1155/2016/4768541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Puig, D., Manzanilla E. G., Morales J., Borda E., Pérez J. F., Piñeiro C., and Chetrit C.. . 2007. Dietary nucleotide supplementation reduces occurrence of diarrhoea in early weaned pigs. Livest. Sci. 108:276–279. doi: 10.1016/j.livsci.2007.01.099 [DOI] [Google Scholar]
- NRC. . 2012. Nutrient Requirements of Swine, 11th ed.; Washington, DC (USA): National Academies Press. [Google Scholar]
- Pan, L., Ma X. K., Zhao P. F., Shang Q. H., Long S. F., Wu Y., and Piao X. S.. . 2018a. Forsythia suspensa extract attenuates breast muscle oxidative injury induced by transport stress in broilers. Poult. Sci. 97:1554–1563. doi: 10.3382/ps/pey012 [DOI] [PubMed] [Google Scholar]
- Pan, L., Zhao P. F., Ma X. K., Shang Q. H., Long S. F., Wu Y., Wang W., and Piao X. S.. . 2018b. Forsythia suspensa extract protects broilers against breast muscle oxidative injury induced by corticosterone mimicked pre-slaughter acute stress. Poult. Sci. 97:2095–2105. doi: 10.3382/ps/pey046 [DOI] [PubMed] [Google Scholar]
- Pan, C. W., Zhou G. Y., Chen W. L., Zhuge L., Jin L. X., Zheng Y., Lin W., and Pan Z. Z.. . 2015. Protective effect of forsythiaside A on lipopolysaccharide/d-galactosamine-induced liver injury. Int. Immunopharmacol. 26:80–85. doi: 10.1016/j.intimp.2015.03.009 [DOI] [PubMed] [Google Scholar]
- Peri, B. A., and Rothberg R. M.. . 1986. Transmission of maternal antibody prenatally and from milk into serum of neonatal rabbits. Immunology 57:49–53. doi: 10.1111/j.1600-065X.1986.tb01474.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao, X. L., Jang M. H., Cui J., and Piao X.. . 2008. Lignans from the fruits of Forsythia suspensa. Bioorg. Med. Chem. Lett. 18: 1980–1984. doi: 10.1016/j.bmcl.2008.01.115 [DOI] [PubMed] [Google Scholar]
- Pluske, J. R. 2013. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 4:1. doi: 10.1186/2049-1891-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluske, J. R., Hampson D. J., and Williams I. H.. . 1997. Factors influencing the structure and function of the small intestine in the nursery pig: a review. Livest. Prod. Sci. 51:215–236. doi: 10.1016/S0301-6226(97)00057-2 [DOI] [Google Scholar]
- Valko, M., Rhodes C. J., Moncol J., Izakovic M., and Mazur M.. . 2006. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160:1–40. doi: 10.1016/j.cbi.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Vondruskova, H., Slamova R., Trckova M., Zraly Z., and Pavlik I.. . 2010. Alternatives to antibiotic growth promoters in prevention of diarrhoea in nursery piglets: a review. Vet. Med. 55:199–224. doi: 10.3906/vet-0908-26 [DOI] [Google Scholar]
- Wang, L., Piao X. L., Kim S. W., Piao X. S., Shen Y. B., and Lee H. S.. . 2008. Effects of Forsythia suspensa extract on growth performance, nutrient digestibility, and antioxidant activities in broiler chickens under high ambient temperature. Poult. Sci. 87:1287–1294. doi: 10.3382/ps.2008-00023 [DOI] [PubMed] [Google Scholar]
- Wang, Y., Zhao H., Lin C., Ren J., and Zhang S.. . 2016. Forsythiaside A exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia cells through activation of Nrf2/HO-1 signaling pathway. Neurochem. Res. 41:659–665. doi: 10.1007/s11064-015-1731-x [DOI] [PubMed] [Google Scholar]
- Wu, G., Bazer F. W., Dai Z., Li D., Wang J., and Wu Z.. . 2014. Amino acid nutrition in animals: protein synthesis and beyond. Annu. Rev. Anim. Biosci. 2:387–417. doi: 10.1146/annurev-animal-022513-114113 [DOI] [PubMed] [Google Scholar]
- Xu, X., Yang C., Chang J., Wang P., Yin Q., Liu C., Gao T., Dang X., and Lu F.. . 2020. Dietary supplementation with compound probiotics and berberine alters piglet production performance and fecal microbiota. Animals 10:511. doi: 10.3390/ani10030511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., Qian K., Wang C., and Wu Y.. . 2018. Roles of probiotic Lactobacilli inclusion in helping piglets establish healthy intestinal inter-environment for pathogen defense. Probiotics Antimicrob. Proteins 10:243–250. doi: 10.1007/s12602-017-9273-y [DOI] [PubMed] [Google Scholar]
- Yin, J., Li Y., Han H., Chen S., Gao J., Liu G., Wu X., Deng J., Yu Q., Huang X., . et al. 2018. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high fat diet-fed mice. J. Pineal. Res. 65:12524. doi: 10.1111/jpi.12524 [DOI] [PubMed] [Google Scholar]
- Yin, J., Ren W., Liu G., Duan J., Yang G., Wu L., Li T., and Yin Y.. . 2013. Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radic. Res. 47:1027–1035. doi: 10.3109/10715762.2013.848277 [DOI] [PubMed] [Google Scholar]
- Yuan, S., Chen D., Zhang K., and Yu B.. . 2007. Effects of oxidative stress on growth performance, nutrient digestibilities and activities of antioxidative enzymes of weanling pigs. Asian-Australas. J. Anim. Sci. 20:1600–1605. doi: 10.5713/ajas.2007.1600 [DOI] [Google Scholar]
- Zeng, Z. K., Li Q. Y., Piao X. S., Liu J. D., Zhao P. F., X. Xu, Zhang S., and Niu S.. . 2014. Forsythia suspensa extract attenuates corticosterone-induced growth inhibition, oxidative injury, and immune depression in broilers. Poult. Sci. 93:1774–1781. doi: 10.3382/ps.2013-03772 [DOI] [PubMed] [Google Scholar]
- Zhang, H. Y., Piao X. S., Zhang Q., Li P., Yi J. Q., Liu J. D., Li Q. Y., and Wang G. Q.. . 2013. The effects of Forsythia suspensa extract and berberine on growth performance, immunity, antioxidant activities, and intestinal microbiota in broilers under high stocking density. Poult. Sci. 92:1981–1988. doi: 10.3382/ps.2013-03081 [DOI] [PubMed] [Google Scholar]
- Zhao, P. Y., Jung J. H., and Kim I. H.. . 2012. Effect of mannan oligosaccharides and fructan on growth performance, nutrient digestibility, blood profile, and diarrhea score in weanling pigs. J. Anim. Sci. 90:833–839. doi: 10.2527/jas.2011-3921 [DOI] [PubMed] [Google Scholar]
- Zhao, P. F., Piao X. S., Zeng Z. K., Li P., Xu X., and Wang H. L.. . 2017a. Effect of Forsythia suspensa extract and chito-oligosaccharide alone or in combination on performance, intestinal barrier function, antioxidant capacity and immune characteristics of nursery piglets. Anim. Sci. J. 88:854–862. doi: 10.1111/asj.12656 [DOI] [PubMed] [Google Scholar]
- Zhao, P. F., Piao X. S., Zeng Z. K., Li Q. Y., Xu X., and Wang H. L.. . 2017b. Forsythia suspensa extract attenuates lipopolysaccharide-induced inflammatory liver injury in rats via promoting antioxidant defense mechanisms. Anim. Sci. J. 88:873–881. doi: 10.1111/asj.12717 [DOI] [PubMed] [Google Scholar]