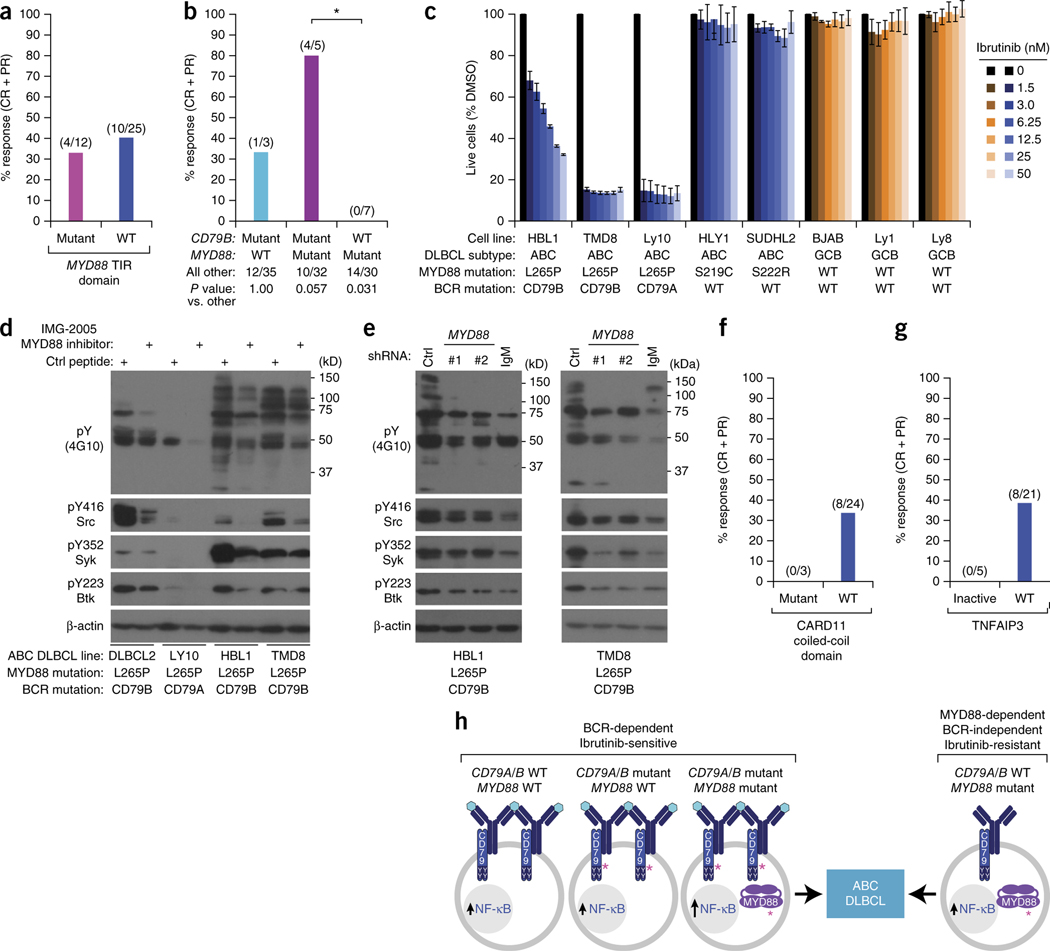
Figure 3.
Influence of recurrent genetic alterations on ibrutinib response in ABC DLBCL. (a) Overall response rates by MYD88 mutation status. Fisher’s exact test of the overall response rate between the MYD88 mutant and non-mutant group (P = 0.493). Fractions above the bars represent the number of subjects with a response (complete or partial response; numerator) over the total number of subjects in the group (denominator). (b) Overall response rates by CD79B and MYD88 mutation status. Fisher’s exact test of the overall response rate between the CD79B mutant/MYD88 mutant group and the CD79B wild type/MYD88 mutant group (*P = 0.01). (c) Toxicity of ibrutinib for cell-line models of ABC and GCB DLBCL harboring genetic lesions in CD79A, CD79B and MYD88, as indicated. Cells were treated for 3 d with ibrutinib at the indicated concentrations and assessed for viability as described5. Error bars denote s.e.m. of triplicates. (d) MYD88 potentiates chronic active BCR signaling in ABC DLBCL. The indicated ABC DLBCL lines were treated with the MYD88 dimerization inhibitor IMG-2005 (100 μM) or a control peptide for 16 h and analyzed by immunoblot for the indicated proteins. Data are representative of three biological repeats. (e) MYD88 knockdown reduces chronic active BCR signaling in ABC DLBCL. TMD8 and HBL1 ABC DLBCL cells were transduced with the indicated shRNAs, induced to express the shRNAs with doxycycline for 48 h, and evaluated by immunoblot analysis for the indicated proteins. Data are representative of independent experiments in HBL1 (n = 7) and TMD8 (n = 10). (f) Overall response rates by CARD11 mutation status. Fractions above the bars represent the number of subjects with a response (complete or partial response; numerator) over the total number of subjects in the group (denominator). (g) Overall response rates by TNFAIP3 status. TNFAIP3 inactivation denotes TNFAIP3 nonsense or frameshift mutation, TNFAIP3 double deletion, or TNFAIP3 mRNA <2 s.d. below the mean of ABC DLBCL samples. TNFAIP3 WT denotes cases without these TNFAIP3 alterations. Fractions above the bars represent the number of subjects with a response (complete or partial response; numerator) over the total number of subjects in the group (denominator). (h) Theoretical model of ABC DLBCL pathogenesis indicating BCR-dependent and BCR-independent genetic pathways. Shown at the left are three genetic scenarios that can be associated with chronic active BCR signaling and ibrutinib sensitivity. Hypothetically, the BCRs in these tumors could be engaged by an antigen (turquoise hexagons) and hence are clustered. The right of this figure illustrates ibrutinib-resistant ABC DLBCL tumors that do not rely on chronic active BCR signaling, but rather use a mutant MYD88 isoform to engage the NF-κB pathway. The BCRs in these tumors are therefore depicted as unclustered and not engaged by an antigen. Pink asterisks (*) indicate activating mutations. ABC, activated B cell–like; BCR, B cell receptor; CR, complete response; DLBCL, diffuse large B cell lymphoma; PR, partial response.