Figure 1.
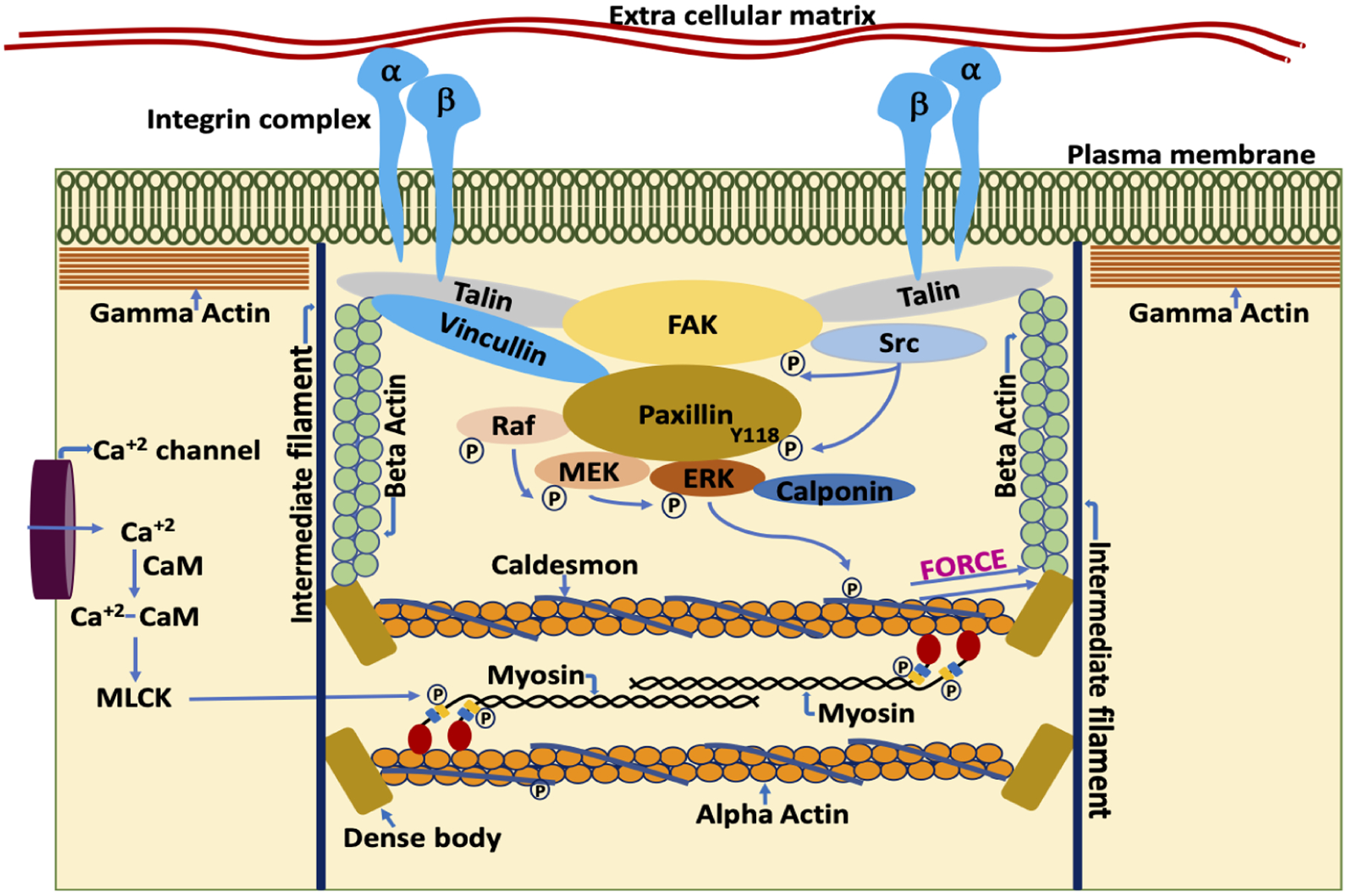
Schematic representation of components of the VSMC by which it regulates contractility and stiffness. The smooth muscle cell plasma membrane is spanned by FA complexes containing, among many other proteins, talin, vinculin, FA kinase (FAK), and integrins [52]. Cytoskeletal proteins of the FA complexes connect to the membrane-spanning integrins, composed of alpha and beta integrin heterodimers. On the cytoplasmic side, the FAs connect to the actin cytoskeletal filaments. Thus, the integrin complex connects the interior of the cell to the ECM allowing cell-matrix communication and signaling [53, 54]. Contractile force is generated by the actomyosin cross bridge cycle. During the cross-bridge cycle, force is generated by movement of myosin head domains while they are attached to the actin filaments. Acto-myosin cross bridge cycling is a tightly regulated process, involving both the thin and thick filaments. Thin filament regulation, in part, involves the blocking of myosin attachment sites on F-actin by caldesmon [55]. Caldesmon, in turn, is regulated by a complex, Src dependent signaling cascade. Src dependent phosphorylation of paxillin at Y118 allows the binding of rapidly accelerated fibrosarcoma (Raf) and extracellular signal-regulated kinase (ERK) to mitogen-activated protein kinase kinase (MEK) bound paxillin [56]. The formation of this complex leads to the activation of MEK by Raf and ERK transphosphorylation by active MEK. Subsequently, activated ERK translocates to, and phosphorylates, caldesmon [57]. Once phosphorylated, caldesmon undergoes a conformation change in its structure and no longer blocks the myosin attachment sites on F-actin. This sequence of events, then promotes acto-myosin interaction. However, attachment of the myosin head to F-actin is also regulated by phosphorylation of the myosin regulatory light chain (MLC), leading to additional signaling cascades described as thick filament regulation [58]. For example, increased intracellular calcium levels during agonist-induced opening of calcium channels in the plasmalemma leads to the formation of calcium-calmodulin complexes which then activates myosin light chain kinase (MLCK) [59–61]. Active MLCK then phosphorylates the myosin light chains which activates myosin ATPase activity [62]. Increased myosin ATPase activity leads to a conformational change in the head of myosin and promotes the attachment of myosin to actin in the strong binding conformation. Force generated during acto-myosin interaction is transmitted to dense bodies and through the nonmuscle actin cytoskeleton, to FA complexes, including the transmembrane integrins and, subsequently, to the ECM and the vessel wall [46, 63]. Simultaneous contraction of the VSMCs in the vessel wall leads to vascular constriction, which, when increased in extent or duration also leads to increased pathologies of hypertension and vascular stiffness [46]
