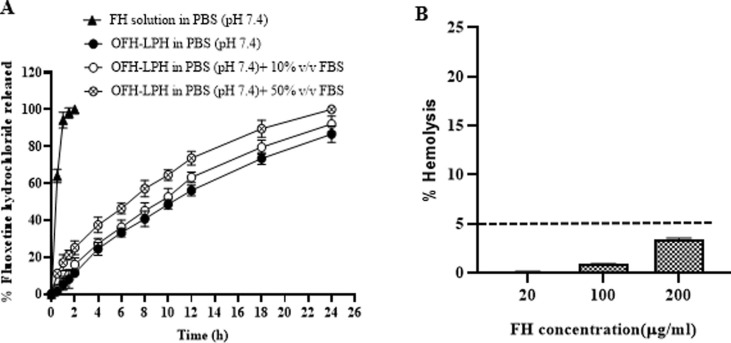
Fig. 9.
In vitro release of FH from FH solution in PBS pH 7.4, OFH-LPH in PBS pH 7.4, OFH-LPH in PBS pH 7.4 in the presence of 10% v/v FBS, and OFH-LPH in PBS pH 7.4 in the presence of 50% v/v FBS (A). Drug concentrations in the dialysate were assessed by HPLC. Datapoint represents mean and SD (n = 3). The presence of 10% v/v FBS gave a similar release pattern to the OFH-LPH, but the presence of 50% v/v FBS increased the release rate of OFH-LPH. The in vitro hemolysis assay of the OFH-LPH (B). Rat RBCs were incubated with OFH-LPH at different FH concentrations (20–200 µg/mL) for 2 h at 37 °C. Positive and negative controls were 0.5% w/v Triton X-100 and PBS (pH 7.4), respectively. Samples were centrifuged at 4000 rpm for 5 min at 4 °C and the absorbance of the released hemoglobin was determined at 545 nm. Datapoint represents mean and SD (n = 3). The dotted line represents the acceptable hemolysis range. OFH-LPH is a biocompatible formula.