Abstract
Women with a history of childhood sexual abuse (CSA) are at greater risk to develop alcohol use disorders (AUDs). While impulsivity has been postulated as a behavioral mechanism linking childhood trauma and alcohol use, few studies have comprehensively examined impulsivity in women with CSA. We compared women with a history of CSA (n= 21) control women who did not endorse CSA or other major traumas (CON; n=21) on self-report measures of impulsivity and risk-taking. Additionally, performance on behavioral impulsivity and subjective response to alcohol were examined before and after acute alcohol (0.00, 0.50, 0.75 g/kg) administration. Overall, women with CSA responded more impulsively than CON women on the Immediate and Delayed Memory Tasks (IMT and DMT; measures of response initiation) and the GoStop task (a measure of response inhibition). While alcohol produced dose-related increases in impulsive responding on the IMT in both groups, alcohol-induced increases in response inhibition on the GoStop task were only evident in the CSA group. In contrast, women with CSA exhibited less risk-taking than the CON group on the Balloon Analogue Risk Task (BART). Alcohol produced dose-related increases on several subjective response measures (e.g., alcohol liking) in both groups; however these ratings tended to be greater in women with CSA. These preliminary data suggest that women with CSA may be more impulsive. Importantly, impulsivity can lead to hazardous drinking and alcohol consumption can further increase impulsivity, putting women with CSA at increased risk for sexual revictimization, particularly in the context of alcohol use.
Keywords: Childhood sexual abuse, Women, Alcohol, Impulsivity, Subjective response
Childhood sexual abuse (CSA) is a prevalent public health issue worldwide (Barth, Bermetz, Heim, Trelle, & Tonia, 2013) that produces enduring neurobiological changes (Heim, Shugart, Craighead, & Nemeroff, 2010) and places victimized individuals at substantial increased risk for developing a wide range of adverse medical, psychological and behavioral problems including depression, posttraumatic stress disorder (PTSD), suicidal behaviors (e.g., Maniglio, 2009; Paolucci, Genuis, & Violato, 2001) and problems with alcohol and other drugs of abuse (Enoch, 2011; Nelson et al., 2006; Sartor, Agrawal, McCutcheon, Duncan, & Lynskey, 2008). Of note, women with a history of CSA appear to be more vulnerable to alcohol and other drug abuse subsequent to trauma exposure than men (Danielson et al., 2009; Kendler et al., 2000; Widom, White, Czaja, & Marmorstein, 2007). Moreover, having a history of CSA, as opposed to other forms of childhood trauma, represents one of the most predictive risk factors for sexual revictimization in adulthood, particularly for women (e.g., Classen, Palesh, & Aggarwal, 2005; Elliott, Mok, & Briere, 2004; Messman-Moore & Long, 2000; but see Werner et al., 2016). This is of further concern since the prevalence rates of CSA reported have typically been 3–5 times higher in women than in men, such that approximately 13–32% of women endorse CSA (Finkelhor, Shattuck, Turner, & Hamby, 2014).
There is converging evidence suggesting that impulsivity, which has been associated separately with both childhood trauma (e.g., Roy, 2005; Somer, Ginzburg, & Kramer, 2012) and alcohol-related problems (e.g., Berey, Leeman, Pittman, & O’Malley, 2017; see reviews by Dick et al., 2010; Lejuez et al., 2010), may be an underlying behavioral mechanism linking childhood trauma and hazardous drinking (Oshri et al., 2018; Schwandt, Heilig, Hommer, George, & Ramchandani, 2013; Shin, Lee, Jeon, & Wills, 2015). With respect to alcohol, moderate to intoxicating doses have been shown to generally increase performance-based measures of impulsivity, particularly rapid-decision tasks and continuous performance tasks, in laboratory studies (Dougherty, Marsh-Richard, Hatzis, Nouvion, & Mathias, 2008; Reed, Levin, & Evans, 2012; Weafer & Fillmore, 2008). Moreover, individuals with more impaired inhibitory control consume more alcohol in the laboratory (Vaughan et al., 2019; Weafer & Fillmore, 2008), demonstrating an association between impulsivity and alcohol use.
When examining impulsivity among individuals with childhood trauma, some studies found that self-reported impulsivity was higher among individuals with childhood trauma (Roy, 2005; Somer et al., 2012). Further, Oshri and colleagues (2018) found that childhood abuse was associated with both self-reported impulsivity and performance on a delay discounting task, whereas Bornovalova, Gwadz, Kahler, Aklin, and Lejuez (2008) found that childhood trauma correlated with a behavioral measure of risk-taking and self-reported sensation seeking, but not self-reported impulsivity. In women with CSA specifically, Navalta, Polcari, Webster, Boghossian, and Teicher (2006) found relatively few differences on impulsive responding using a go/no-go/stop task compared to control women. Similarly, although Meyers et al. (2019) observed an atypical neural response among individuals with CSA during a go/no-go task, behavioral performance on this task was not different relative to groups with other histories of childhood trauma (i.e., non-assaultive trauma or other non-sexual assaultive trauma). Lastly, a mixed gender laboratory study found that individuals with childhood sexual or physical trauma scored higher on self-report measures of impulsivity and risk-taking but exhibited less risk-taking on a behavioral risk task, had fewer false alarms on an emotional go/no-go task and showed no differences on a delay discounting task, relative to controls (Sujan, Humphreys, Ray, & Lee, 2014). The inconsistent findings across these studies examining childhood trauma and impulsivity may be due to a number of factors that differed across studies, including the type of childhood trauma, how trauma was assessed (e.g., self-report questionnaire or interview), the gender composition and the scope of impulsivity measures evaluated.
In an attempt to control for some of mixed results of previous studies, the present study recruited a more homogeneous sample consisting of women (rather than mixed gender samples) who specifically endorsed a history of CSA (as opposed to various types of childhood trauma). Women who primarily endorsed CSA, but did not have any current psychopathology, were recruited to better represent the general population of sexually abused individuals (Copeland, Keeler, Angold, & Costello, 2007; Widom, 1999; Wijma, Söderquist, Björklund, & Wijma, 2000). All participants were carefully screened to determine appropriate group assignment. Specifically, childhood trauma was assessed via a clinical interview rather than self-report questionnaires and a structured clinical interview was conducted to rule out any current psychiatric disorders. Further, the present study assessed several self-report questionnaires of impulsivity, as well as multiple behavioral impulsivity and risk-taking tasks to encompass the multidimensional aspects of impulsivity (see reviews by Dick et al., 2010; Lejuez et al., 2010). Although these two constructs are related to one another (Reynolds, Ortengren, Richards, & de Wit, 2006a), risk-taking is not necessarily a core dimension of impulsivity (Lejuez et al., 2010).
As mentioned above, women with CSA are more likely to engage in a number of impulsive behaviors such as hazardous or binge drinking and sexual risk taking. This also puts them at increased risk for sexual revictimization (Elliott et al., 2004; Messman-Moore & Long, 2000; Ullma Najdowski, & Filipas, 2009), often in the context of alcohol use (Parkhill, Norris, & Davis, 2014; Walsh et al., 2013; Wilhite, Mallard, & Fromme, 2018; but see Messman-Moore & Long, 2002). To more comprehensively evaluate alcohol-induced increases in behavioral impulsivity among women with CSA, the present study also assessed the response to acute doses of alcohol under controlled laboratory conditions among women with CSA compared to a group of control (CON) women who did not endorse any history of CSA or other major childhood traumas.
In addition to the impulsivity measures, subjective response measures sensitive to the effects of alcohol were included since both increased positive or hedonic subjective responses and reduced negative subjective responses to alcohol have been associated with an increased risk for alcohol use disorders (see reviews by Quinn & Fromme, 2011; Ray, Bujarski, & Roche, 2016). Further, there is some evidence that a differential subjective response to alcohol is one mechanism linking impulsivity or impaired control to increased risk for drinking (Berey et al., 2017; Leeman et al., 2014; Vaughan et al., 2019; Wardell, Quilty, & Hendershot, 2015). For instance, among nondependent social drinkers, those who were more impulsive (based on a self-report questionnaire) reported less sedation and greater stimulation from alcohol using a controlled intravenous alcohol administration procedure. The present study was designed to extend the current literature by assessing both behavioral impulsivity and the subjective response to alcohol during acute alcohol administration specifically among women with CSA. We hypothesized that compare to the CON group, the CSA group would: 1) be more impulsive based on both self-report and behavioral measures of impulsivity; 2) exhibit more impulsive responding after alcohol; and 3) report greater positive subjective effects from alcohol.
Method
Participants
Advertisements in local media were aimed to recruit equal sample sizes of women with a history of CSA and healthy control women to participate in a research study examining the effects of various non-prescription or prescription drugs, or alcohol on mood and task performance. The Institutional Review Board of the New York State Psychiatric Institute approved the study. Participants gave their written informed consent before beginning the study and were financially compensated for their participation.
Based on over 1,500 detailed telephone interviews, 91 women completed the initial in-person screening. Of those, 49 women met full criteria and started the study, but 7 women discontinued due to schedule conflicts. Enrollment continued until an equal number of women in each group completed the study. Thus, all analyses were based on the 42 completers (21 CSA group and 21 CON group). All women were medically and psychiatrically healthy based on a physical examination, a structured clinical interview, 12-lead electrocardiogram, clinical blood chemistries, and urinalyses. None of the women were pregnant, nursing or had been pregnant within the past six months. All women were normally cycling and were not using hormonal contraceptives or other prescription medications. No one met criteria for any current DSM-IV-TR Axis I psychiatric disorders (American Psychiatric Association, 2000) within the last year, including psychoactive substance abuse or dependence (other than nicotine) based on the Structured Clinical Interview for DSM-IV (SCID I, First, Spitzer, Gibbon, & Williams, 2002). With respect to lifetime psychiatric disorders, only 3 women in the CON group met criteria for one or more past disorders including alcohol abuse (3) and depression (1). Eleven women in the CSA group met criteria for one or more past disorders including depression (6), PTSD (3), alcohol abuse (3), bulimia (2), cannabis abuse (1) and obsessive-compulsive disorder (1).
Trauma History.
The Early Trauma Inventory, a 56-item clinician-administered interview (ETI; Bremner, Vermetten, & Mazure, 2000) was used to assess the presence or absence of childhood trauma, including sexual, physical, emotional and general trauma. During the interview the clinician also asked about any history of adult trauma. While self-report is the most common approach for assessing childhood trauma, this can result in a slight risk of underestimating trauma (Fergusson, Horwood, & Woodward, 2000), although a recent meta-analysis (Baldwin, Reuben, Newbury, & Danese, 2019) reported that the agreement between prospective and retrospective measures of childhood trauma was higher when the assessment of childhood trauma was based on interviews rather than self-report questionnaires. Women assigned to the CSA group had to endorse a history of CSA, defined as having experienced sexual contact with a person 5 or more years older before the age of 12, irrespective of consent or between the ages of 12–16 years, unless wanted or not distressing at the time. Sexual contact was defined as touching or fondling; attempts to have the child arouse the adult, or touch his/her body in a sexual way; the adult rubbing his/her genitals against the child’s body in a sexual way; touching the child’s genitals with the mouth or having the child touch the adult with her mouth; attempts to have anal or vaginal intercourse with the child; and/or anal or vaginal penetration or intercourse. Women in the CSA group could also endorse other childhood traumas (e.g., physical abuse, emotional abuse), as well as adult sexual assault (ASA). In contrast, women in the CON group were excluded if they reported a history of CSA or ASA, as well as any other major childhood or adult traumas; those who endorsed minimal trauma exposure, conservatively defined as < 2 on any subscale of the ETI, were included given that most individuals endorse some level of trauma (e.g., being ignored, spanked with a hand, shouted at) based on the ETI (Bremner et al., 2000).
Procedures
Alcohol Challenge Sessions.
Women had 5 outpatient sessions conducted on separate days. The first session was a practice session to familiarize participants with the study procedures and provide training on the impulsivity tasks. On the practice session participants received two placebo capsules and a placebo beverage to reduce expectancies related to alcohol administration. The testing phase consisted of three sessions where participants ingested two capsules (always placebo) and alcohol (0.0, 0.50, and 0.75 g/kg), with dose order randomized within and across groups. The last session was a lottery session when participants could potentially get one of their outcomes from the DDT (see Reed et al., 2012 for details). Data from the practice and lottery sessions were not included in the data analysis.
Given that there is some evidence that the response to alcohol can vary across the menstrual cycle (e.g., Evans & Levin, 2011), menstrual cycle phase was controlled for by conducting all alcohol challenge sessions during the mid-follicular phase (days 4–10 after the onset of menstruation) of the menstrual cycle. Participants completed a modified Daily Ratings Form (see Reed et al., 2012) each evening to track changes in mood symptoms across the menstrual cycle, document the onset and duration of menstruation, and record the number of standard alcoholic beverages consumed. Participants contacted the laboratory when they started menstruating. The alcohol administration sessions were spaced a minimum of 24 hours apart to facilitate completing the alcohol administration sessions in the same menstrual cycle. Seventy-four percent of participants (17 in the control group and 14 in the CSA group) were able to complete the alcohol administration sessions during the follicular phase of a single menstrual cycle. For the remaining participants, missed sessions were rescheduled during the follicular phase of the next menstrual cycle. Under those conditions, participants briefly practiced the study procedures, particularly the behavioral impulsivity tasks, before the alcohol administration session began. For both groups, there were no differences in baseline performance on the behavioral tasks across the 3 alcohol administration sessions (ps> 0.43) indicating stable performance across sessions.
Participants reported to the laboratory at 0830 and remained until 1530. Participants were instructed not to eat breakfast before reporting to the laboratory and to refrain from using all psychoactive drugs (with the exception of tobacco, caffeinated products and alcohol) for the duration of the study. Participants were instructed not to drink alcohol the day before a session. To verify that participants were not intoxicated, upon arrival each session a field sobriety test was conducted, a urine specimen was collected and analyzed for the presence of illicit drugs or pregnancy, and a breath alcohol test was conducted (Alco-Sensor III, Intoximeters, Inc., St. Louis, MO). Blood samples were drawn for hormone assays and hormone levels confirmed that sessions were conducted during the follicular phase; estradiol levels were 47.72 (± 8.09) pg/ml and progesterone levels were 0.84 (± 0.08) ng/ml, with no differences between the two groups.
Participants ate a light breakfast (with a caffeinated beverage for those individuals who regularly consumed caffeine to avoid caffeine withdrawal), and then completed a baseline assessment battery (described below). Then participants ingested two capsules and a beverage and completed the assessment battery at specified times for the remainder of the session. After the 3 hr assessment battery, participants were provided lunch. Cigarette smoking was only permitted after lunch to avoid nicotine withdrawal and limit the effects of nicotine on mood and performance. At the end of each session, participants were required to pass a field sobriety test and remain in the laboratory until breath alcohol concentrations were ≤ 20 mg/dl. Participants were provided round trip subway fare and were instructed not to drive, take any medications, or drink alcohol the remainder of the day.
Impulsivity and Risk-Taking Measures.
During screening participants completed three self-report measures of impulsivity: the Barratt Impulsiveness Scale, version 11 (BIS-11; Patton, Stanford, & Barratt, 1995), the Eysenck Impulsivity Questionnaire (EIQ; Eysenck, Pearson, Easting, & Allsopp, 1985), and the Sensation-Seeking Scale (SSS; Zuckerman, Eysenck, & Eysenck, 1978).
During the alcohol challenge sessions, three measures of behavioral impulsivity [1) the Immediate Memory Task/Delayed Memory Task (IMT/DMT; Dougherty & Marsh, 2003), 2) the GoStop task (Dougherty, Mathias, & Marsh, 2003), 3) Delay Discounting Task (DDT; Kirby, Petry, & Bickel, 1999)] and one measure of risk-taking [(the Balloon Analogue Risk Task (BART; Lejuez et al., 2002)] were assessed (see Reed et al., 2012 for details). Each session, the entire impulsivity battery was conducted at baseline, 1, 2 and 4 hrs after alcohol administration and took approximately 40 min, with the exception that the DDT and the BART were also completed at 0.5 hrs after alcohol administration.
The IMT/DMT is a continuous performance task developed by Dougherty and colleagues (Dougherty et al., 2002; Dougherty & Marsh, 2003) that yields a number of measures related to response initiation. In this study participants had one 5-min block of IMT followed by one 5-min block of DMT, with a 30-sec rest period between blocks. In the IMT, a series of 5-digit numbers appeared successively on a computer monitor. Participants were instructed to respond when the stimulus on the monitor was identical to the one that preceded it. Each 5-digit number appeared for 500 msec, and successive numbers were separated by a 500-msec inter-trial interval. The DMT required participants to remember a 5-digit number and then compare it with another that was presented 3.5 sec later. During the 3.5-sec interval, repetitive distracter stimuli (the 5-digit number 12345) were presented at the same rate and duration as the other stimuli. Participants were instructed to ignore the distracter stimuli and to remember and compare only the numbers spanning the distracter stimuli. While the IMT and DMT each yield several dependent measures (correct detections, commission errors, and response latencies), the IMT ratio and DMT ratio (defined as the proportion of commission errors to correct detections) have been considered the primary dependent measure of impulsivity (Dougherty et al., 2002; Dougherty et al., 2008), with higher ratios indicating higher levels of impulsivity.
The GoStop Task is a task that measures response inhibition (Dougherty et al., 2003). In this task, a series of 5-digit numbers in black on a white background were presented, with randomly generated 5-digit numbers appearing for 500 msec every 2 sec (500 msec on, 1500 msec off). Participants were told to respond when the number they saw was identical to the previous number. Half of all target trials featured a target-stop trial when the color of the matching target’s numerals changed from black to red at 50, 150, 250 and 350 msec after its presentation. Participants were instructed to respond to the identically matching numbers before the number disappeared from the screen, but not to respond to a number that turned red. The number of target-stop stimuli that the participant did not respond to was used to calculate the percentage of inhibited responses from the total presentations for each delay condition. The GoStop ratio (i.e., the number of response inhibition failures relative to the number of responses to go trials) has been shown to be a valid measure of inhibitory responding, without the confound of reaction time, which can be affected by alcohol (Dougherty et al., 2009), with higher ratios indicating higher levels of impulsivity. In this study, the primary dependent measure of response inhibition was the GoStop ratio for the 150-msec delay because this delay has been shown to be most sensitive to detecting the effects of alcohol (Dougherty et al., 2008) and differences between groups (Marsh et al., 2002).
The DDT measures the devaluation of future rewards and the version we used consisted of a fixed set of 27 choices between smaller immediate rewards and larger delayed rewards (Kirby et al., 1999); reward values ranged between $11-$85 and delays ranged between 7 days to 6 months. The primary dependent measure was the average k value, which determines the discount rate, or the steepness of the reduction in the present value of a reward with increases in delay to that reward. Higher k values indicate higher levels of impulsivity. Participants were informed that on their last session (the lottery session), they would role a die; if they rolled a 6, one of their choices on the DDT was selected and implemented (e.g., if they selected $33 in 14 days, the money was given when the time had elapsed).
The BART (Lejuez et al., 2002) is a behavioral measure of risk-taking. This task involves displaying a small balloon on a computer screen. Each pump on the balloon was accompanied by an accrual of 5 cents and the average pump break point was 64 pumps. If the balloon was pumped past its individual explosion point, a “pop” sound was generated and all money in the temporary bank was lost, then the next uninflated balloon was displayed. At any point during each balloon trial, the participant could stop pumping the balloon and click the collect money button that would transfer all money from the temporary bank to the permanent bank. Fifteen balloon trials were presented each time. The primary dependent measure was the number of adjusted pumps, defined as the average number of pumps excluding balloons that exploded (i.e. the average number of pumps on each balloon before money collection); higher scores indicate greater risk-taking. Participants received a percentage (20%) of the money earned on the BART and this was paid upon study completion.
Subjective Response Measures.
The Biphasic Alcohol Effects Scale (BAES; Martin, Earleywine, Musty, Perrine, & Swift, 1993) is a 14-item rating scale that provides measures of alcohol’s effects on an 11-point scale from 0 to 10. The BAES results in two subscales measuring the stimulant (BAES Stimulation) and sedative (BAES Sedation) effects of alcohol. The Drug Effects Questionnaire (DEQ; Evans & Levin, 2004) asked participants to rate the drug effects they were experiencing (e.g., Good Drug Effect, Willing to Take Again) on a 5-point scale from 0–4, with the exception that ratings of drug/alcohol liking were measured on a 9-point scale from −4 to 4 (see Reed et al., 2012 for details). These measures were completed at 0.5, 1, 2, 3, 4 and 5 hrs after alcohol administration, with the exception that the BAES was also completed at baseline.
Alcohol.
To reduce alcohol expectancies, participants were informed that they could receive placebo, over-the-counter medications, prescription medications or alcohol but they would never receive two active medications on the same day. A double-dummy design was employed such that participants ingested two placebo capsules (size 0 gelatin capsules filled with lactose powder) and a 350 ml beverage (0.00, 0.50, 0.75 g/kg alcohol) each session with alcohol dose calculated based on the estimated total body water of each participant (see Evans & Levin, 2004 for details). The beverage consisted of tonic water and cranberry juice, with 100 proof Absolut ® vodka added to achieve the correct volume for each individualized alcohol dose. The placebo and active beverages for a given individual were isocaloric (no more than a 10-calorie difference between beverages), using regular or low calorie tonic and juice and dextrose or Equal sweetener. In order to further mask the content of the beverage, each beverage was topped with 1 ml of vodka and 1 drop of peppermint oil. Participants had 5 min to consume the entire beverage (placebo or alcohol) and capsules under the supervision of an investigator.
Data Analyses
Analyses were based on the 42 women who completed the entire study (21 CON and 21 CSA). Student’s t-tests, chi-square tests and Fisher’s exact tests were used to compare demographic characteristics, current patterns of alcohol and other drug use, histories of trauma, and self-report measures of impulsivity between the CON and CSA groups. Cohen’s d and 95% confidence intervals for the unstandardized mean differences were calculated.
For the alcohol administration sessions, the peak (i.e., maximal) value at 1–2 hours after dosing for each participant for each measure after alcohol administration was determined and analyzed. For all measures, separate mixed model repeated measures analyses of variance (ANOVA) using SPSS Version 26 software (Mac SPSS, Inc., Chicago, IL) were conducted with Group (CON vs. CSA) as the between-subjects factor and Dose (0.00, 0.50, 0.75 g/kg) as the within-subjects factors. Planned contrasts were used to compare across doses within each group and to compare the two groups at each dose. For all analyses, results were considered statistically significant if p < 0.05 and to reduce the potential for Type I errors, Huynh-Feldt corrections were used. Effect sizes were reported as partial eta squared calculations.
Results
Demographics
The two groups were well matched on most demographic characteristics (Table 1). Although women in the CSA group had significantly higher depression scores at screening compared to the CON group, these differences were not clinically significant (Beck, Steer, Ball, & Ranieri, 1996). Since numerous studies have shown that level of alcohol use can enhance impulsivity (Dougherty et al., 2008; Reed et al., 2012; Weafer & Fillmore, 2008), concerted efforts were made to match the CON group to the CSA group on drinking level. Based on the prospective daily ratings, this was successfully accomplished since there were no significant differences in alcohol consumption between the two groups (Table 1). While no one in the CON group smoked tobacco cigarettes or smoked cannabis in the past month, 6 women the CSA group smoked tobacco cigarettes daily (averaging 9 cigarettes per day) and 4 women in the CSA group reported smoking cannabis in the previous month. However, none of the women in either group had a positive breath alcohol test or any positive urine drug screen, including cannabis, on any of the laboratory sessions.
Table 1.
Demographic Characteristics of Participants
| Control Group (n = 21) | CSA Group (n = 21) | Significance | Cohen’s d | 95% CI | |
|---|---|---|---|---|---|
|
| |||||
| Age (years) | 28.57 (4.20) | 29.10 (6.53) | 0.76 | −0.10 | (−3.95, 2.90) |
| Race (White/Black/Hispanic/Other) | 12 /2 /3 /4 | 9 /5 /3 /4 | p<0.6 | ||
| Body Mass Index (kg/m2) | 22.27 (1.93) | 22.87 (3.34) | 0.48 | −0.22 | (−2.29, 1.10) |
| Education (years) | 16.24 (1.37) | 15.60 (1.53) | 0.16 | 0.44 | (−0.26; 1.55) |
| Beck Depression Inventory Score | 2.81 (3.88) | 6.33 (5.62) | <0.03 | −.073 | (−6.53, −0.51) |
| Menstrual Cycle Length (days) | 28.58 (2.10) | 27.98 (2.28) | 0.38 | 0.27 | (−0.77, 1.97) |
| Recreational Drug Use | |||||
| Alcohol (drinks/week) | 6.83 (5.11) | 9.26 (4.61) | 0.11 | −0.50 | (−5.46, 0.61) |
| Range (drinks/week) | 1.0 – 16.5 | 1.0 – 17.0 | |||
| Cigarette Smokers (n) | 0 | 6 | p<0.02 | ||
| (cigarettes/day) | 0.0 | 9.0 | p<0.03 | −2.75 | (−16.68, −1.32) |
| Cannabis (n) | 0 | 4 | p=0.11 | ||
| Early Trauma Inventory | |||||
| Total Early Trauma Score | 3.71 (3.12) | 12.19 (5.34) | p<0.0001 | −1.94 | (−11.23, −5.73) |
| General | 1.24 (1.64) | 2.57 (1.60) | p<0.02 | −0.82 | (−2.34, −.32) |
| Physical | 1.19 (1.08) | 2.62 (1.77) | p<0.003 | −0.97 | (−2.35, −0.51) |
| Emotional | 1.29 (1.55) | 2.29 (2.28) | 0.11 | −0.52 | (−2.22, 0.22) |
| Sexual | 0.00 (0.00) | 4.71 (2.53) | p<0.0001 | −2.63 | (−5.87, −3.56) |
All demographics are presented as means (+/− SD) unless otherwise denoted.
Quantitative data were tested with independent t-tests and frequency data were tested with Fisher’s exact tests. Cigarette smokers were defined as those smoking cigarettes daily and cannabis smokers were defined as those reporting smoking cannabis in the past month.
Based on the ETI (Table 1), the CSA group scored significantly higher than the CON group on total trauma scores and 3 types of early trauma, with sexual abuse (the trauma of interest) endorsed by all participants in the CSA group. In addition, 6 women in the CSA group (29%) reported being sexually assaulted as an adult (ASA). While histories of CSA and ASA were exclusionary in the CON group, Table 1 shows that the CON group did report low levels of general, physical and emotional abuse during childhood.
Impulsivity and Risk-Taking
Table 2 shows that there were minimal differences on the three impulsivity self-report questionnaires completed during screening. The CSA group tended to have higher total BIS-11 scores (p< 0.08) and higher scores on the Experience Seeking subscale of the SSS-V (p< 0.07). Otherwise, there were no statistically significant differences between the CSA group and the CON group on the total scores or subscale scores of the BIS-11, the EIQ, and the SSS-V.
Table 2.
Self-report Impulsivity Questionnaires
| Control Group (n = 21) | CSA Group (n = 21) | Significance a | Cohen’s d | 95% CI | |
|---|---|---|---|---|---|
|
| |||||
| Barratt Impulsivity Scale (BIS-11) | |||||
| Total Impulsivity Score | 59.71 (9.35)b | 64.90 (9.39) | p < 0.08 | −0.55 | (−11.03, 0.65) |
| Attentional | 14.00 (3.33) | 15.57 (3.22) | 0.13 | −0.48 | (−3.61, 0.47) |
| Motor | 20.19 (3.57) | 21.24 (3.13) | 0.32 | −0.32 | (−3.14, 1.05) |
| Non-planning | 25.52 (4.75) | 28.10 (5.91) | 0.13 | −0.48 | (−5.91, 0.77) |
| Eysenck Impulsivity Questionnaire (EIQ) | |||||
| Total Impulsivity Score | 21.19 (5.03) | 22.62 (6.27) | 0.42 | −0.25 | (−4.97, 2.12) |
| Impulsiveness | 4.24 (2.76) | 5.48 (3.57) | 0.22 | −0.39 | (−3.23, 0.75) |
| Venturesomeness | 7.52 (3.64) | 7.05 (2.48) | 0.62 | 0.15 | (−1.48, 2.43) |
| Empathy | 9.43 (2.69) | 10.10 (2.23) | 0.39 | −0.27 | (−2.21, 0.88) |
| Zuckerman Sensation-Seeking Scale (SSS-V) | |||||
| Total Sensation-Seeking Score | 20.62 (7.37) | 21.95 (5.94) | 0.52 | −0.20 | (−5.51, 2.84) |
| Thrill & Adventure Seeking | 6.71 (3.07) | 5.81 (2.56) | 0.31 | 0.32 | (−0.85, 2.67) |
| Experience Seeking | 6.19 (2.18) | 7.43 (2.04) | < 0.07 | −0.59 | (−2.56, 0.08) |
| Disinhibition | 4.71 (2.59) | 5.38 (2.29) | 0.38 | −0.27 | (−2.19, 0.86) |
| Boredom Susceptibility | 3.00 (1.92) | 3.33 (2.61) | 0.64 | −0.15 | (−1.76, 1.10) |
Data comparing the two groups were tested with independent t-tests; CI = confidence interval.
Mean data with standard deviations shown in parentheses.
Figure 1 shows the results based on peak behavioral impulsivity and risk-taking tasks during the alcohol challenge sessions as a function of group and alcohol dose. The CSA group exhibited significantly more impulsive responding than the CON group based on the IMT ratio [Group effect: F(1,40) = 14.05, p = 0.001, η2p=0.26], the DMT ratio [Group effect: F(1,40) = 24.76, p < 0.0001, η2p=0.38], and the GoStop 150 msec ratio [Group effect: F(1,40) = 12.54, p = 0.001, η2p=0.24], after placebo and both doses of alcohol (based on planned contrasts between the two groups at each dose, all ps < 0.01). Alcohol produced dose-related increases on the IMT ratio [Dose effect: F(2,40) = 13.31, p < 0.0001, η2p=0.25] in both groups. Based on planned contrasts, for both groups the low dose of alcohol did not differ from placebo (ps>0.11), whereas the high dose of alcohol was significantly greater than placebo (ps<0.005); also the high dose of alcohol was significantly greater than the low dose of alcohol (ps<0.03). This was true for both groups, but for all doses (including placebo), the IMT ratio was significantly greater in the CSA group. Although alcohol also produced dose-related increases on the GoStop 150 msec ratio [Dose effect: F(2,40) = 7.52, p < 0.001, η2p=0.16], this increased impulsivity was driven by the CSA group [Dose x Group interaction: F(2,80) = 4.05, p < 0.02, η2p=0.09]. For the GoStop ratio, planned contrasts showed that both the low dose (p<0.006) and high dose of alcohol (p<0.005) were significantly greater in the CSA group relative to placebo, but there was no difference between the two active doses of alcohol (p>0.62). In contrast, the GoStop ratio did not vary as a function of alcohol dose in the CON group (ps>0.23). There was no effect of alcohol dose on the DMT [Dose effect: F(2,40) = 1.64, p > 0.20] and there were no significant Group effects [F(1,40) = 1.24, p > 0.27], Dose effects [F(2,40) = 2.22, p > 0.13] or Dose x Group interactions on the DDT [F(2,80) = 0.49, p > 0.56]. In contrast, risk-taking on the BART was significantly lower in the CSA group compared to the CON group [Group effect: F(1,40) = 4.37, p = 0.043, η2p=0.10]; based on the planned contrasts this was evident following placebo and 0.5 g/kg alcohol (ps < 0.05). However, there were no significant Dose effects [F(2,40) = 0.35, p > 0.69] or Dose x Group interactions on the BART [F(2,80) = 0.27, p > 0.74].
Fig. 1.

Peak impulsive responding on the Immediate Memory Task (IMT; expressed as a ratio), Delayed Memory Task (DMT; expressed as a ratio), the 150 msec GoStop Task (expressed as a ratio), the Delay Discounting Task (DDT; overall k value), and the Balloon Analogue Risk Task (BART; adjusted number of pumps) as a function of group and alcohol dose. Bars show means (CON group =21; CSA group = 21) + 1 S.E.M. * with bracket denotes a significant difference between groups (p ≤ 0.05) at that dose. Based on the planned contrasts within the CSA group, # denotes a significant difference between placebo and the high dose of alcohol and ## denotes a significant difference between the low dose and high dose of alcohol. Based on the planned contrasts within the CON group, ^ denotes a significant difference between placebo and the high dose of alcohol and ^^ denotes a significant difference between the low dose and high dose of alcohol.
Subjective Response Measures
Figure 2 shows that alcohol produced significant dose-related increases in peak ratings of Alcohol Liking [Dose effect: F(2,80) = 20.41, p < 0.0001, η2p=0.34] and the CSA group tended to report greater liking for alcohol than the CON group [Group effect: F(1,40) = 3.16, p = 0.083, η2p=0.07]. Similarly, dose-related effects were observed for ratings of Willing to Take Again [Dose effect: F(2,80) = 6.14, p < 0.01, η2p=0.13] and there was a trend for this to be greater in the CSA group [Dose x Group interaction: F(2,80) = 2.95, p = 0.063, η2p=0.07]. While alcohol produced dose-related increases in ratings of Good Effect [Dose effect: F(2,80) = 25.63, p < 0.0001, η2p=0.39; data not shown], there were no significant differences between the two groups (p >0.16). As also shown in Figure 2, while alcohol produced the expected increases in BAES sedation scores [Dose effect: F(2,40) = 20.59, p < 0.0001, η2p=0.34], these increases were primarily driven by the CSA group [Group effect: F(1,40) = 4.36, p < 0.05 η2p=0.10]. Although there was no main Group effect [F(1,40) = 0.86, p > 0.35, η2p=0.02] or Dose effect [F(2,40) = 2.13, p > 0.13, η2p=0.05] on BAES Stimulation scores, the CSA group reported higher scores than the CON group [Dose x Group interaction: F(2,80) = 4.88, p < 0.02, η2p=0.11] after alcohol. Based on planned contrasts, Stimulation scores for both the low dose and high dose of alcohol were significantly greater relative to placebo in the CSA group (ps < 0.04), but there was no difference between the two active doses of alcohol (p > 0.90). In contrast, in the CON group, Stimulation scores after either dose of alcohol did not differ from placebo (ps > 0.17).
Fig. 2.
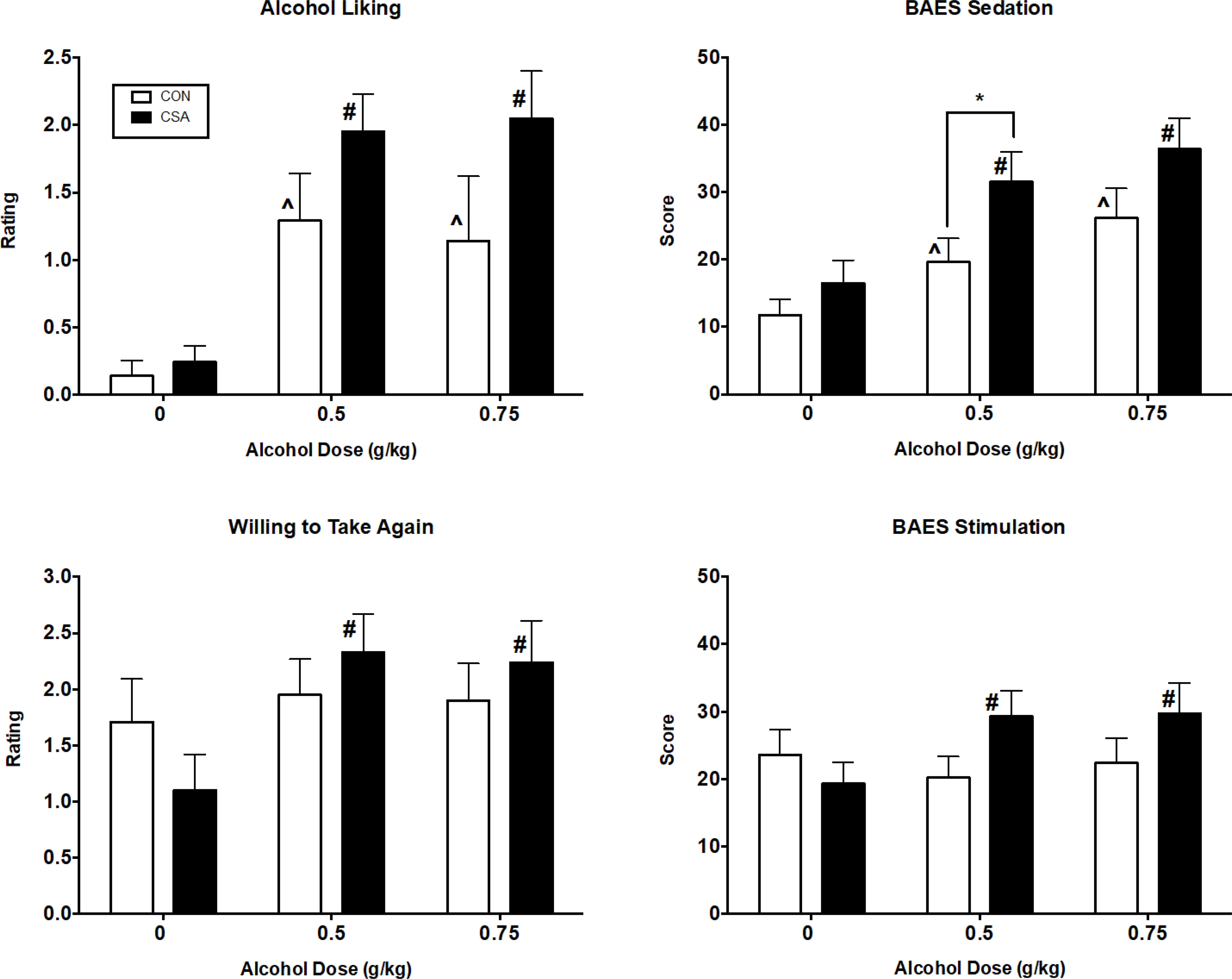
Peak subjective response effects for ratings of Alcohol Liking, Willing to Take Again, BAES Sedation scores and BAES Stimulation scores as a function of group and alcohol dose. See Fig. 1 for details.
Other Measures
Alcohol produced significant dose-related increases in peak breath alcohol levels [Dose effect: F(2,80) = 1158.00; p <0.0001, η2p=0.97], reaching a maximum of 58 and 91 mg/dl following 0.50 and 0.75 g/kg alcohol, respectively approximately 1 hr after alcohol administration, with no differences between the two groups. Alcohol also produced small, but statistically significant dose-related increases in maximal heart rate [Dose effect: F(2,80) = 6.81; p <0.01, η2p=0.15] and diastolic blood pressure [Dose effect: F(2,80) = 3.53; p <0.04, η2p=0.08], with no differences between the two groups (ps >.0.09). There were no significant changes in systolic blood pressure as a function of alcohol dose or group (ps > 0.59).
Discussion
To our knowledge this pilot study is the first to comprehensively examine impulsivity and the effects of alcohol on behavioral impulsivity in women with a common childhood trauma of CSA under controlled laboratory conditions. The most important finding in this study is that women with CSA, but without any current psychiatric disorders, responded more impulsively than the CON group on several behavioral impulsivity tasks (i.e., the IMT/DMT and the GoStop tasks) in the absence of alcohol administration (i.e., when placebo was administered). Our findings contrast with two previous studies that assessed go/no-go task performance among individuals with histories of childhood trauma. In a large study conducted among adolescents and young adults with histories of childhood trauma compared to those without childhood trauma, Meyers et al. (2019) failed to observe any behavioral differences on a go/no-go stop task. Using an emotional go/no-go task, Sujan et al. (2014) reported that young adults with a history of childhood physical or sexual abuse actually were less impulsive in that they made fewer false alarms relative to controls. However, both of these studies were conducted in mixed gender samples of adolescents or young adults, and childhood trauma was broadly defined and inclusive. On the other hand, among a sample of college women who only endorsed a history of CSA, Navalta et al. (2006) found modest evidence that women with CSA exhibited impaired inhibitory responding on a go/no-go stop task relative to a group of control women. Our current findings provide additional support suggesting that women with CSA have impaired inhibitory control.
While the results with the IMT/DMT and the GoStop behavioral tasks support our initial hypothesis that women in the CSA group would be more impulsive than the CON group, self-report measures of impulsivity and risk-taking did not differ substantially between the CSA and CON groups. There are several potential explanations for why our findings are divergent from previous studies that found higher trait impulsivity among individuals with childhood trauma (Roy et al., 2005; Somer et al., 2012; Sujan et al., 2014). First, the sample size of the current pilot study was relatively small, which may have hindered our statistical power to detect group differences. Second, our CSA group consisted entirely of adult women, all who endorsed a history of CSA but had minimal histories of other childhood traumas, and they were devoid of any current mental health disorders. In addition, in the present study the two groups were matched on average alcohol consumption and this may have limited our ability to detect group differences. For instance, previous studies have observed that self-report measures of impulsivity are greater among heavy at-risk drinkers relative to light drinkers (e.g., MacKillop, Mattson, MacKillop, Castelda, & Donovick, 2007; Reed et al., 2012). We also did not observe any differences between the two groups on the DDT (a measure of devaluation of future rewards), whereas when we assessed risk-taking behavior using the BART, women in the CSA group actually exhibited less risk-taking behavior compared to women in the CON group. Despite a younger and more heterogeneous group, Sujan et al. (2014) also reported no differences on delay discounting and less risk-taking on the BART among young adults with a history of childhood physical or sexual abuse compared to individuals with no history of childhood abuse. While Bornovalova et al. (2008) reported a correlation between childhood abuse and increased risk-taking using a youth version of the BART, that study was conducted among low-income African American adolescents and did not directly compare traumatized youth to a control group.
We also hypothesized that the CSA group would exhibit more impulsive responding and risk-taking after alcohol administration. This was only partially supported by two behavioral impulsivity tasks (the IMT and the GoStop Task) showing dose-related increases in behavioral impulsivity after alcohol. This is consistent with previous studies that have also shown alcohol-induced increases in impulsive responding on the IMT in social drinkers (Dougherty et al., 2008; Reed et al., 2012; Weafer & Fillmore, 2008); in the present study, the CSA group was more impulsive than the CON group independent of alcohol dose. While previous studies in social drinkers have failed to observe alcohol-related impairment of response inhibition using GoStop or Go/No-Go tasks (Dougherty et al., 2008; Guillot, Fanning, Bullock, McCloskey, & Berman, 2010; Reed et al., 2012; Reynolds, Richards, & de Wit, 2006b), in the present study, not only was the CSA group more impulsive than the CON group overall, but alcohol-induced increases in response inhibition were evident only in the CSA group. While previous studies administered similar doses of alcohol as the present study, one major difference is that most of them failed to assess histories of childhood trauma. However, Reed et al. (2012) also did not observe alcohol-related increases in GoStop responding among female moderate drinkers but importantly, those participants were carefully screened to rule out any major history of trauma, suggesting that a history of childhood trauma may be an important factor in alcohol-induced impaired inhibitory control.
As mentioned above, DDT performance did not differ between the two groups, even after alcohol administration, which is consistent with the limited number of studies that measured delay discounting in individuals with a history of childhood trauma (Oshri et al., 2018; Sujan et al., 2014). Previous studies in both social and heavy drinkers have also reported mixed results with the DDT (Field, Christiansen, Cole, & Goudie, 2007; MacKillop et al., 2007; Reed et al., 2012; Reynolds et al., 2006b). Lastly, the fact that alcohol did not alter performance on the BART is consistent with a previous study (Reynolds et al., 2006b). Taken together, our findings also suggest that behavioral measures of impulsivity, particularly performance tasks that measure response initiation and response inhibition, may be more sensitive than either discounting or risk-taking measures in detecting impairment among individuals with CSA. The results of this pilot study provide converging evidence that impulsivity may be a mediating link between CSA and problematic drinking since some behavioral measures of impulsivity were increased after alcohol administration.
Similar to previous studies (Evans & Levin, 2011; Holdstock, King, & de Wit, 2000; Reed et al., 2012; Vaughan et al., 2019), alcohol administration increased several subjective effects. Further alcohol-induced subjective ratings of Alcohol Liking, Willing to Take Again and Stimulation scores tended to be higher in the CSA group relative to the CON group. To some extent this supports our initial hypotheses, but we were underpowered to detect more robust differences between the two groups. This is most likely attributable to the fact that the two groups were carefully matched on their level of drinking given that previous studies have reported that moderate/heavy social drinkers have a greater positive subjective response to alcohol compared to light drinkers (Holdstock et al., 2000; Reed et al., 2012). Regardless, drinking at a moderate level and/or having a history of CSA may confer enhanced risk for problematic alcohol use. Unfortunately, this was not able to be determined in the present study since participants were not given the opportunity to self-administer alcohol in the laboratory as has been done by others (Weafer & Fillmore, 2008; Vaughan et al., 2019).
This study had several strengths, including the careful screening of participants, testing all women during the same phase of the menstrual cycle, administering more than one dose of alcohol and assessing a range of self-report and behavioral measures to capture the multidimensional construct of impulsivity. Another potential strength is that we specifically recruited women who endorsed a history of CSA in order to have a common history of childhood trauma and excluded individuals with any current psychopathology. Only 14% (3 of 21) of the CSA group endorsed a past history of PTSD, which is consistent with previous reports indicating that the majority of individuals with a history of childhood trauma, including CSA, do not develop PTSD, or may only have PTSD at some point in their lifetime, with few remaining symptomatic into adulthood (Copeland, et al., 2007; Widom, 1999; Wijma, et al., 2000).
This pilot study also had several limitations that need to be considered when interpreting the findings and generalizing to the community at large. First, the major limitation is that although power estimates based on the extant literature suggested that we would have adequate sample sizes and power, the present study was actually insufficiently powered to detect more robust differences between the two groups. The failure to have adequate power in the present study is unclear. The most parsimonious explanation is that, with the exception of the CSA group having a history of childhood sexual abuse, our groups did not differ on other variables, such as level of drinking or current psychiatric disorders, which was intentional to control for potential confounds.
A second limitation is that only women were recruited although the prevalence of CSA among men is higher than previously assumed (Barth et al., 2013; Werner et al., 2016) and should be included in future research. Further, other forms of childhood trauma, such as physical abuse, were not exclusionary. Although women with CSA were explicitly recruited for the CSA group, given the high prevalence of overlapping risk factors among women with CSA, it would have been extremely difficult to recruit a sample of CSA women devoid of any other trauma exposure. In fact, Navalta et al. (2006) found that only 10% of college women recruited with CSA had no other histories of trauma. We also did not exclude women with ASA in the CSA group and a surprising 29% of the CSA group reported ASA, but we did not have a sufficient sample size to explore the role of ASA. Further research is needed given that revicitimization as an adult is not only associated with CSA (Classen et al., 2005), but also problem drinking (Messman-Moore & Long, 2002; Ullman, Najdowski, & Filipas, 2009).
In summary, the results of this small controlled laboratory study suggest that even in a presumably low-risk resilient sample (i.e., no current psychopathology or problematic alcohol use), women with a history of CSA may be more impulsive and that under some conditions alcohol consumption can further increase impulsivity. Increased alcohol use may further increase other impulsive behaviors, including high-risk sexual behaviors, which can increase the risk for sexual revictimization, particularly in the context of alcohol use (Messman-Moore & Long, 2002; Ullman et al., 2009). Thus, individuals may be caught in a cycle of escalating alcohol use and trauma exposure that increases the risk for a range of adult psychiatric disorders including alcohol use disorders, depression, and PTSD. Although the present findings are intriguing, they need to be confirmed in a larger sample. In addition, further research is needed to determine if the impulsive behavior among CSA women we observed actually results in increased alcohol use and if there are differences between men and women with histories of CSA and other childhood traumas.
Public Health Significance.
Women with childhood sexual abuse (CSA), but without any current psychopathology, exhibit more behavioral impulsivity under baseline conditions than healthy control women. Alcohol also decreased inhibitory control. These data have important implications for women with CSA since impulsivity often leads to hazardous drinking and alcohol consumption can further increase impulsivity and increase the risk for sexual revictimization. Further research in a larger sample is needed to examine if impulsive behavior among CSA women increases alcohol use and if there are sex differences among individuals with histories of CSA and other childhood traumas.
DISCLOSURES AND ACKNOWLEDGEMENTS
This research was supported by National Institute on Drug Abuse (NIDA) grants R01DA009114 (Evans) and K01DA022282 (Reed). had no role other than financial support. All authors contributed in a significant way to the manuscript and all authors have read and approved the final manuscript. All the authors declare that they have no conflicts of interest to report that could inappropriately influence or be perceived to influence this work. The authors gratefully acknowledge the assistance of Dr. Frances R. Levin and the numerous research and clinical staff who helped conduct various aspects of this study.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: Author. [Google Scholar]
- Baldwin JR, Reuben A, Newbury JB, & Danese A (2019). Agreement between prospective and retrospective measures of childhood maltreatment: A systematic review and meta-analysis. JAMA Psychiatry, 76, 586–593. 10.1001/jamapsychiatry.2019.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth J, Bermetz L, Heim E, Trelle S, & Tonia T (2013). The current prevalence of child sexual abuse worldwide: A systematic review and meta-analysis. International Journal of Public Health, 58, 469–483. 10.1007/s00038-012-0426-1 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, & Ranieri WF (1996). Comparison of Beck Depression Inventories-IA and -II in Psychiatric Outpatients. Journal of Personality Assessment, 67, 588–597. 10.1207/s15327752jpa6703_13 [DOI] [PubMed] [Google Scholar]
- Berey BL, Leeman RF, Pittman B, & O’Malley SS (2017). Relationships of impulsivity and subjective response to alcohol use and related problems. Journal of Studies on Alcohol and Drugs, 78, 835–843. 10.15288/jsad.2017.78.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Gwadz MA, Kahler C, Aklin WM, & Lejuez CW (2008). Sensation seeking and risk-taking propensity as mediators in the relationship between childhood abuse and HIV-related risk behavior. Child Abuse & Neglect, 32, 99–109. 10.1016/j.chiabu.2007.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, & Mazure CM (2000). Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: The early trauma inventory. Depression & Anxiety, 12, 1–12. [DOI] [PubMed] [Google Scholar]
- Classen CC, Palesh OG, & Aggarwal R (2005). Sexual revictimization: A review of the empirical literature. Trauma, Violence, & Abuse, 6, 103–129. 10.1177/1524838005275087 [DOI] [PubMed] [Google Scholar]
- Copeland WE, Keeler G, Angold A, & Costello EJ (2007). Traumatic events and posttraumatic stress in childhood. Archives of General Psychiatry, 64, 577–584. 10.1001/archpsyc.64.5.577 [DOI] [PubMed] [Google Scholar]
- Danielson CK, Amstadter AB, Dangelmaier RE, Resnick HS, Saunders BE, & Kilpatrick DG (2009). Trauma-related risk factors for substance abuse among male vs female young adults. Addictive Behaviors, 34, 395–399. 10.1016/j.addbeh.2008.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, & Sher K (2010). Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology, 15, 217–226. 10.1111/j.1369-1600.2009.00190.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM & Marsh DM (2003). IMT/DMT: A research tool for studying attention, memory, and impulsive behavior (Version 2.0) [Manual]. Houston, TX: Neurobehavioral Research Laboratory and Clinic, University of Texas Health Science Center at Houston. [Google Scholar]
- Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, & Mathias CW (2008). A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug and Alcohol Dependence, 96, 111–120. 10.1016/j.drugalcdep.2008.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, & Marsh DM (2003). GoStop impulsivity paradigm (version 1.0) [Manual]. Houston, TX: Neurobehavioral Research Laboratory and Clinic, University of Texas Health Science Center at Houston. [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Furr RM, Nouvion SO, & Dawes MA (2009). Distinctions in behavioral impulsivity: Implications for substance abuse research. Addictive Disorders & Their Treatment, 8, 61–78. DOI: 10.1097/ADT.0b013e318172e488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DM, Mok DS, & Briere J (2004). Adult sexual assault: Prevalence, symptomatology, and sex differences in the general population. Journal of Traumatic Stress, 17, 203–211. 10.1023/B:JOTS.0000029263.11104.23 [DOI] [PubMed] [Google Scholar]
- Enoch MA (2011). The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology, 214, 17–31. 10.1007/s00213-010-1916-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, & Levin FR (2004). Differential response to alcohol in light and moderate female social drinkers. Behavioural Pharmacology, 15, 167–181. doi: 10.1097/01.fbp.0000131575.38620.a5 [DOI] [PubMed] [Google Scholar]
- Evans SM, & Levin FR (2011). Response to alcohol in women: Role of the menstrual cycle and a family history of alcoholism. Drug and Alcohol Dependence, 114, 18–30. 10.1016/j.drugalcdep.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck SBG, Pearson PR, Easting G, & Allsopp JF (1985). Age norms for impulsiveness, venturesomeness and empathy in adults. Personality and Individual Differences, 6, 613–619. 10.1016/0191-8869(85)90011-X [DOI] [Google Scholar]
- Fergusson DM, Horwood LJ, & Woodward LJ (2000). The stability of child abuse reports: A longitudinal study of the reporting behaviour of young adults. Psychological Medicine, 30, 529–544. 10.1017/S0033291799002111 [DOI] [PubMed] [Google Scholar]
- Field M, Christiansen P, Cole J, & Goudie A (2007). Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction, 102, 579–586. 10.1111/j.1360-0443.2007.01743.x [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Shattuck A, Turner HA, & Hamby SL (2014). The lifetime prevalence of child sexual abuse and sexual assault assessed in late adolescence. Journal of Adolescent Health, 55, 329–333. 10.1016/j.jadohealth.2013.12.026 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (2002). Structured clinical interview for DSM-IV Axis I disorders, Research Version, Patient Edition. (SCID-I/P, Version 3.0). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Guillot CR, Fanning JR, Bullock JS, McCloskey MS, & Berman ME (2010). Effects of alcohol on tests of executive functioning in men and women: A dose response examination. Experimental and Clinical Psychopharmacology, 18, 409–417. 10.1037/a0021053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, & Nemeroff CB (2010). Neurobiological and psychiatric consequences of child abuse and neglect. Developmental Psychobiology, 52, 671–690. 10.1002/dev.20494 [DOI] [PubMed] [Google Scholar]
- Holdstock L, King AC, & de Wit H (2000). Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcoholism Clinical & Experimental Research, 24, 789–794. 10.1111/j.1530-0277.2000.tb02057.x [DOI] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, & Prescott CA (2000). Childhood sexual abuse and adult psychiatric and substance use disorders in women: An epidemiological and cotwin control analysis. Archives of General Psychiatry, 57, 953–959. 10.1001/archpsyc.57.10.953 [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, & Bickel WK (1999). Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology: General, 128, 78–87. 10.1037//0096-3445.128.1.78 [DOI] [PubMed] [Google Scholar]
- Leeman RF, Ralevski E, Limoncelli D, Pittman B, O’Malley SS, & Petrakis IL (2014). Relationships between impulsivity and subjective response in an IV ethanol paradigm. Psychopharmacology, 231, 2867–2876. 10.1007/s00213-014-3458-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, & de Wit H (2010). Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcoholism Clinical & Experimental Research, 34, 1334–1345. 10.1111/j.1530-0277.2010.01217.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, . . . Brown RA (2002). Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART). Journal of Experimental Psychology: Applied, 8, 75–84. 10.1037//1076-898x.8.2.75 [DOI] [PubMed] [Google Scholar]
- MacKillop J, Mattson RE, MacKillop EJA, Castelda BA, & Donovick PJ (2007). Multidimensional assessment of impulsivity in undergraduate hazardous drinkers and controls. Journal of Studies on Alcohol and Drugs, 68, 785–788. 10.15288/jsad.2007.68.785 [DOI] [PubMed] [Google Scholar]
- Maniglio R (2009). The impact of child sexual abuse on health: A systematic review of reviews. Clinical Psychology Review, 29, 647–657. 10.1016/j.cpr.2009.08.003 [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, & Swift RM (1993). Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clinical & Experimental Research, 17, 140–146. 10.1111/j.1530-0277.1993.tb00739.x [DOI] [PubMed] [Google Scholar]
- Marsh DM, Dougherty DM, Mathias CW, Moeller FG, & Hicks LR (2002). Comparisons of women with high and low trait impulsivity using behavioral models of response-inhibition and reward-choice. Personality and Individual Differences, 33, 1291–1310. 10.1016/S0191-8869(02)00014-4 [DOI] [Google Scholar]
- Messman-Moore TL, & Long PJ (2000). Child sexual abuse and revictimization in the form of adult sexual abuse, adult physical abuse, and adult psychological maltreatment. Journal of Interpersonal Violence, 15, 489–502. 10.1177/088626000015005003 [DOI] [Google Scholar]
- Messman-Moore TL, & Long PJ (2002). Alcohol and substance use disorders as predictors of child to adult sexual revictimization in a sample of community women. Violence and Victims, 17, 319–340. 10.1891/vivi.17.3.319.33662 [DOI] [PubMed] [Google Scholar]
- Meyers J, McCutcheon VV, Pandey AK, Kamarajan C, Subbie S, Chorlian D, . . . Porjesz B (2019). Early sexual trauma exposure and neural response inhibition in adolescence and young adults: Trajectories of frontal theta oscillations during a go/no-go task. Journal of the American Academy of Child & Adolescent Psychiatry, 58, 242–255. 10.1016/j.jaac.2018.07.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navalta CP, Polcari A, Webster DM, Boghossian A, & Teicher MH (2006). Effects of childhood sexual abuse on neuropsychological and cognitive function in college women. Journal of Neuropsychiatry and Clinical Neurosciences, 18, 45–53. 10.1176/jnp.18.1.45 [DOI] [PubMed] [Google Scholar]
- Nelson EC, Heath AC, Lynskey MT, Bucholz KK, Madden PAF, Statham DJ, & Martin NG (2006). Childhood sexual abuse and risks for licit and illicit drug-related outcomes: A twin study. Psychological Medicine, 36, 1473–1483. 10.1017/S0033291706008397 [DOI] [PubMed] [Google Scholar]
- Oshri A, Kogan SM, Kwon JA, Wickrama KAS, Wanderbroek L, Palmer AA, & MacKillop J (2018). Impulsivity as a mechanism linking childhood abuse and neglect with substance use in adolescence and adulthood. Development and Psychopathology, 30, 417–435. 10.1017/S0954579417000943 [DOI] [PubMed] [Google Scholar]
- Paolucci EO, Genuis ML, & Violato C (2001). A meta-analysis of the published research on the effects of child sexual abuse. The Journal of Psychology, 135, 17–36. 10.1080/00223980109603677 [DOI] [PubMed] [Google Scholar]
- Parkhill MR, Norris J, & Davis KC (2014). The role of alcohol use during sexual situations in the relationship between sexual revictimization and women’s intentions to engage in unprotected sex. Violence and Victims, 29, 492–505. 10.1891/0886-6708.vv-09-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, & Barratt ES (1995). Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology, 51, 768–774. 10.1002/1097-4679(199511) 51:6<768::AID-JCLP2270510607>3.0.CO;2-1 [DOI] [PubMed] [Google Scholar]
- Quinn PD, & Fromme K (2011). Subjective response to alcohol challenge: A quantitative review. Alcoholism Clinical and Experimental Research, 35, 1759–1770. 10.1111/j.1530-0277.2011.01521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, & Roche DJO (2016). Subjective response to alcohol as a research domain criterion. Alcoholism Clinical and Experimental Research, 40, 6–17. 10.1111/acer.12927 [DOI] [PubMed] [Google Scholar]
- Reed SC, Levin FR, & Evans SM (2012). Alcohol increases impulsivity and abuse liability in heavy drinking women. Experimental and Clinical Psychopharmacology, 20, 454–465. 10.1037/a0029087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, & de Wit H (2006a). Dimensions of impulsive behavior: Personality and behavioral measures. Personality and Individual Differences, 40, 305–315. 10.1016/j.paid.2005.03.024 [DOI] [Google Scholar]
- Reynolds B, Richards JB, & de Wit H (2006b). Acute-alcohol effects on the Experiential Discounting Task (EDT) and a question-based measure of delay discounting. Pharmacology Biochemistry and Behavior, 83, 194–202. 10.1016/j.pbb.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Roy A (2005). Childhood trauma and impulsivity. Possible relevance to suicidal behavior. Archives of Suicide Research, 9, 147–151. 10.1080/13811110590903990 [DOI] [PubMed] [Google Scholar]
- Sartor CE, Agrawal A, McCutcheon VV, Duncan AE, & Lynskey MT (2008). Disentangling the complex association between childhood sexual abuse and alcohol-related problems: A review of methodological issues and approaches. Journal of Studies on Alcohol and Drugs, 69, 718–727. 10.15288/jsad.2008.69.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt ML, Heilig M, Hommer DW, George DT, & Ramchandani VA (2013). Childhood trauma exposure and alcohol dependence severity in adulthood: Mediation by emotional abuse severity and neuroticism. Alcoholism Clinical and Experimental Research, 37, 984–991. 10.1111/acer.12053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SH, Lee S, Jeon S-M, & Wills TA (2015). Childhood emotional abuse, negative emotion-driven impulsivity, and alcohol use in young adulthood. Child Abuse and Neglect, 50, 94–103. 10.1016/j.chiabu.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somer E, Ginzburg K, & Kramer L (2012). The role of impulsivity in the association between childhood trauma and dissociative psychopathology: Mediation versus moderation. Psychiatry Research, 196, 133–137. 10.1016/j.psychres.2011.08.010 [DOI] [PubMed] [Google Scholar]
- Sujan AC, Humphreys KL, Ray LA, & Lee SS (2014). Differential association of child abuse with self-reported versus laboratory-based impulsivity and risk-taking in young adulthood. Child Maltreatment, 19, 145–155. 10.1177/1077559514543827 [DOI] [PubMed] [Google Scholar]
- Ullman SE, Najdowski CJ, & Filipas HH (2009). Child sexual abuse, post-traumatic stress disorder, and substance use: Predictors of revictimization in adult sexual assault survivors. Journal of Child Sexual Abuse, 18, 367–385. 10.1080/10538710903035263 [DOI] [PubMed] [Google Scholar]
- Vaughan CL, Stangl BL, Schwandt ML, Corey KM, Hendershot CS, & Ramchandani VA (2019). The relationship between impaired control, impulsivity, and alcohol self-administration in nondependent drinkers. Experimental and Clinical Psychopharmacology, 27, 236–246. 10.1037/a0026463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K, Messman-Moore T, Zerubavel N, Chandley RB, DeNardi KA, & Walker DP (2013). Perceived sexual control, sex-related alcohol expectancies and behavior predict substance-related sexual revictimization. Child Abuse and Neglect, 37, 353–359. 10.1016/j.chiabu.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell JD, Quilty LC, & Hendershot CS (2015). Alcohol sensitivity moderates the indirect associations between impulsive traits, impaired control over drinking, and drinking outcomes. Journal of Studies on Alcohol and Drugs, 76, 278–286. 10.15288/jsad.2015.76.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, & Fillmore MT (2008). Individual differences in acute alcohol impairment of inhibitory control predict ad libitum alcohol consumption. Psychopharmacology, 201, 315–324. 10.1007/s00213-008-1284-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner KB, McCutcheon VV, Challa M, Agrawal A, Lynskey MT, Conroy E, . . . Nelson EC (2016). The association between childhood maltreatment, psychopathology, and adult sexual victimization in men and women: results from three independent samples. Psychological Medicine, 46, 563–573. 10.1017/S0033291715002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS (1999). Posttraumatic stress disorder in abused and neglected children grown up. The American Journal of Psychiatry, 156, 1223–1229. 10.1176/ajp.156.8.1223 [DOI] [PubMed] [Google Scholar]
- Widom CS, White HR, Czaja SJ, & Marmorstein NR (2007). Long-term effects of child abuse and neglect on alcohol use and excessive drinking in middle adulthood. Journal of Studies on Alcohol and Drugs, 68, 317–326. 10.15288/jsad.2007.68.317 [DOI] [PubMed] [Google Scholar]
- Wijma K, Söderquist J, Björklund I, & Wijma B (2000). Prevalence of post-traumatic stress disorder among gynecological patients with a history of sexual and physical abuse. Journal of Interpersonal Violence, 15, 944–958. 10.1177/088626000015009003 [DOI] [Google Scholar]
- Wilhite ER, Mallard T, & Fromme K (2018). A longitudinal event-level investigation of alcohol intoxication, alcohol-related blackouts, childhood sexual abuse, and sexual victimization among college students. Psychology of Addictive Behaviors, 32, 289–300. 10.1037/adb0000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Eysenck S, & Eysenck HJ (1978). Sensation seeking in England and America: Cross-cultural, age, and sex comparisons. Journal of Consulting and Clinical Psychology, 46, 139–149. 10.1037//0022-006x.46.1.139 [DOI] [PubMed] [Google Scholar]


