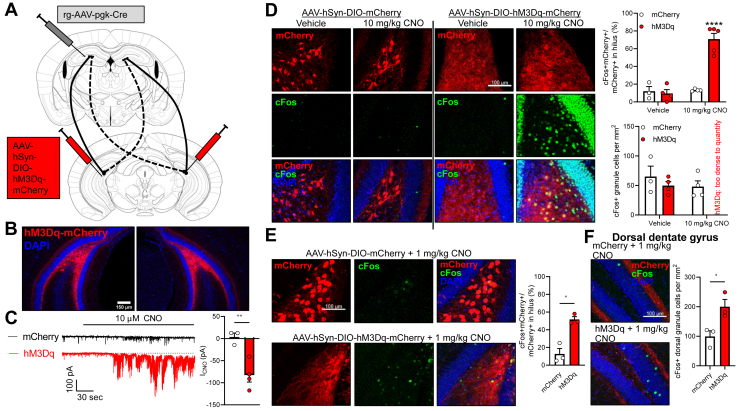
Figure 4.
Chemogenetic activation of vMCs activates dorsal granule cells. (A) To express hM3Dq-mCherry or mCherry in vMCs, retrograde-AAV-pgk-Cre was unilaterally infused into dDG, and AAV-hSyn-DIO-hM3D(Gq)-mCherry or AAV-hSyn-DIO-mCherry was bilaterally infused into vDG hilus. (B) Fluorescence microscopy showing representative image of bilateral vMC targeting. (C) Whole-cell recordings from vMCs expressing either mCherry (3 cells from 2 mice) or hM3Dq-mCherry (4 cells from 3 mice) revealed that hM3Dq+ neurons showed significantly greater mean inward current after bath application of 10 micromolar CNO than control mCherry+ neurons. (t5 = 4.24, ∗∗p = .0068). (D) Mice with vMCs expressing mCherry (n = 3–4 mice) or hM3Dq-mCherry (n = 4–5 mice) were administered vehicle or 10 mg/kg CNO i.p., then perfused 90 minutes later and immunostained for c-Fos. Only vMCs expressing hM3Dq-mCherry strongly expressed c-Fos, but also strongly expressed c-Fos in neighboring vDG granule cells (treatment × virus: F1,12 = 33.38, p < 10−4, ∗∗∗∗p < 10−4 vs. all other groups). (E) Same method as in panel (D) but with reduced dose of CNO (1 mg/kg) showed increase in c-Fos in vMCs expressing hM3Dq compared with mCherry (n = 3 mice per group; t2.98 = 5.58, ∗p = .012), but no hyperactivation of neighboring granule cells. (F) dDG granule cell c-Fos was also significantly upregulated in mice with vMC expression of hM3Dq-mCherry treated with 1 mg/kg CNO as compared with mice with vMC expression of mCherry (n = 3 mice per group; t3.77 = 3.21, ∗p = .036). CNO, clozapine N-oxide; dDG, dorsal dentate gyrus; vMC, ventral mossy cell; vDG, ventral DG.