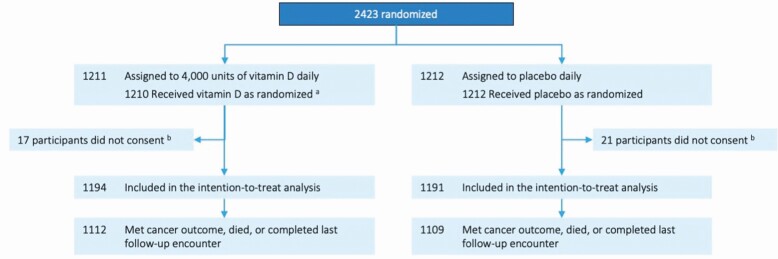
Figure 1.
Flow of participants through the D2dCA study. a One participant did not receive study pills because the participant was randomized with Urine Albumin Creatinine Ratio above the eligibility safety threshold. b Participants did not consent to the D2d cancer ancillary study because the cancer protocol was not approved at two sites.