Abstract
Introduction
As the FDA works to determine whether a nicotine reduction policy would benefit public health, one key question is whether to mandate an immediate or gradual reduction in nicotine levels in cigarettes. The aim of this study was to determine whether the effects of gradual versus immediate nicotine reduction on cigarettes per day (CPD), total nicotine equivalents, and subjective responses differed in younger adults versus older adults.
Methods
Using data from a recent randomized trial conducted in the United States (N = 1250) that switched smokers over a 20-week period to very low nicotine content (VLNC) cigarettes either immediately, gradually (via monthly reductions in nicotine content), or not at all (control condition, normal nicotine content research cigarette), we analyzed the moderating effect of age (age 18–24 or 25+).
Results
For both age groups, CPD in the immediate condition was significantly lower relative to gradual condition (estimated mean difference of 6.3 CPD in young adults, 5.2 CPD in older adults; p’s < .05). Younger and older adults in the immediate and gradual reduction conditions had lower total nicotine equivalents at Week 20 (all p’s < .05) than those in the control condition; age group did not moderate this effect. Positive subjective responses to cigarettes were lower among young adults relative to older adults in the immediate condition.
Conclusions
These results indicate that an immediate reduction in nicotine would result in beneficial effects in both young and older adults. Young adults show less positive subjective effects of smoking following switching to VLNC cigarettes relative to older adults.
Implications
As researchers work to understand how a potential reduced-nicotine product standard for cigarettes may affect public health, one question is whether nicotine should be reduced immediately or gradually. This study demonstrates that both young and older adults who were switched immediately to the lowest content of nicotine smoked fewer CPD and had lower nicotine intake than those in the gradual condition. Furthermore, young adults appear to show lower positive subjective effects following switching to VLNC cigarettes relative to older adults. This is consistent with previous work demonstrating that young people appear to show lower abuse liability for VLNC cigarettes.
Introduction
Cigarette smoking remains the leading cause of death in the United States, with 480 000 Americans dying annually because of combustible cigarette use.1,2 To reduce the public health toll of these products, the FDA has announced its intention to reduce the level of nicotine that is allowable in commercial cigarettes.3 A reduced-nicotine product standard would reduce the addictiveness of cigarettes and is theorized to shift current smokers away from combustible cigarettes and toward cessation.4 Large clinical trials have demonstrated that in adults, switching from usual brand to very low nicotine content (VLNC) cigarettes results in reduced cigarette consumption and toxicant exposure.5,6
One key question concerning the introduction of a reduced-nicotine standard for cigarettes is whether to enact such a standard by immediately reducing nicotine content of cigarettes to a very low level or by gradually reducing the level of nicotine over time. A recent large randomized, double-blind clinical trial (N = 1250) compared these two methods of nicotine reduction across 20 weeks of exposure.7 In the immediate group, participants were switched to 0.4 mg/g cigarettes at the start of the 20 weeks. In the gradual group, participants were given cigarettes that were reduced in nicotine content each month until 0.4 mg/g cigarettes were given at Week 16. The control group smoked normal nicotine content (15.5 mg/g) research cigarettes for all 20 weeks. At Week 20, participants in the immediate reduction group smoked fewer cigarettes per day (CPD) and had lower levels of tobacco-related toxicants and nicotine dependence relative to both the gradual and control groups.
These promising results indicate that immediate reduction is likely to lead to the most beneficial health outcomes in the general population of adults. However, within the general population, some important subgroups may be differentially affected by this policy. One important group is young adults (defined here as ages 18–24, consistent with prior work).8 Young adult smokers differ from their older counterparts in that while they show the highest overall prevalence of any tobacco use relative to other age categories, they tend to smoke fewer CPD, smoke more intermittently, and report less severe nicotine dependence.9–11 Furthermore, young peoples’ motives for smoking are heavily influenced by their peers.12,13 Finally, young adults necessarily have shorter histories of smoking14 and show less stimuli-induced craving.15,16
Young adults also respond to VLNC cigarettes differently than older adults. Using data from a 6-week trial of immediate reduction to reduced nicotine content (2.4–0.4 mg/g) cigarettes,5 we found that young adults in the reduced nicotine content VLNC group showed lower positive subjective evaluations of the study cigarettes after 2 weeks of use relative to older adults in the VLNC group.17 Young adults also smoked fewer CPD than older adults after 2 weeks. After 6 weeks, however, cigarette use had also diminished in the older adult group such that differences across age groups were no longer significant. As subjective responses are an index of abuse liability, these results suggest that VLNC cigarettes may have lower abuse liability in young adults early in the transition to this product standard, but that differences across age groups may diminish over time.
Given these differences between young and older adults, we tested whether the effects of immediate adults gradual reduction in nicotine content were moderated by age (18–24 or 25+) using data from the clinical trial described above.7 The aim of this study was to assess effects on cigarette use and nicotine intake after 20 weeks of study cigarette exposure to determine whether the primary effects of immediate adults gradual condition differed by age. Next, in keeping with our previous work, we also assessed whether subjective responses to VLNC cigarettes at Week 20 were different across age groups within each condition to determine whether young adults showed less positive subjective responses to the VLNC cigarettes relative to older adults. We hypothesized that, in line with our previous work, young adults would show greater reductions in CPD in VLNC groups and greater negative responses to VLNC cigarettes response relative to older adults; however, we did not anticipate a differential reaction to the nicotine reduction schedule (eg, immediate vs gradual) in young adults relative to older adults.
Methods
The current study is a secondary analysis of data collected in a large, double-blind randomized controlled trial conducted at 10 sites across the United States. Full methods and primary results are available in the parent study.7
Participants
A total of 1250 participants across 10 sites in the United States were randomized in a 2:2:1 ratio of experimental conditions to control and included in the analysis (N = 87 young adults, N = 1163 older adults). A 2:2:1 ratio was chosen to maximize the power for detecting significant differences between the experimental groups, which were expected to be smaller than differences between the experimental and control conditions. Participants were recruited from the local communities using a variety of methods including newspaper ads, direct mailings, online advertisements, and radio ads. To be eligible for inclusion, participants had to be daily smokers (at least five cigarettes smoked per day and expired breath CO of >8 parts per million or urine NicAlert level of 6) over the age of 18 with no uncontrolled medical or psychiatric conditions. Participants who used other tobacco products such as e-cigarettes or cigarillos on more than 9 days of the past 30 days were excluded, as were those participants who used only roll-your-own cigarettes. Participants who expressed intentions to quit smoking within the next month were also excluded, as were those participants who were pregnant, trying to conceive, or currently breastfeeding. Participants were also required to take a urine toxicological screen; those who tested positive for drug use (not including marijuana) were also excluded.
Baseline Phase
Eligible participants underwent a 2-week period of smoking their usual brand cigarettes. Each day, participants completed phone calls reporting the number of cigarettes they smoked the day prior using an interactive voice response (IVR) system. Participants visited the lab to complete assessments once per week.
Experimental Phase
Following the baseline phase, participants were randomized in blocks using a double-blind procedure across sites to one of three experimental cigarette groups. From that point on, participants were asked to use only their assigned study cigarettes and none of their usual brand. Participants received free study cigarettes at each session throughout the study. At each in-person session, they received study cigarette compliance counseling. In addition, a semibogus pipeline was employed to increase compliance in which participants were asked to give urine samples at each visit and were told that up to four randomly selected samples would be tested to confirm study cigarette compliance, which would be reinforced with a monetary bonus at the follow-up visit. In actuality, only samples at weeks 18 and 20 from participants in the immediate and gradual conditions were tested to determine bonuses. All participants in the control condition received bonuses. Participants were assigned to either menthol or nonmenthol cigarettes depending on their usual brand preference. Participants visited the lab weekly for the first month of the study; following their Week 4 session, visits occurred every other week.
Immediate Reduction Group
Following the baseline phase, participants in the immediate reduction condition (n = 503) were provided with cigarettes with very low levels of nicotine (0.4 mg/g nicotine) for the 20-week experimental phase.
Gradual Reduction Group
Following the baseline phase, participants in the gradual condition (n = 498) were assigned to smoke research cigarettes that decreased in nicotine content every 4 weeks. For the first 4 weeks, participants smoked a cigarette containing usual levels of nicotine (15.8 mg/g). At their Week 4 lab visit, participants were asked to return their cigarettes and were given cigarettes containing 11.7 mg/g of nicotine. The cigarettes provided were progressively lower in nicotine at Week 8 (5.3 mg/g) and Week 12 (2.4 mg/g). At the Week 16 session, participants were provided the lowest nicotine content cigarettes (0.4 mg/g), which were the same cigarettes the immediate group had been receiving since Week 1.
Control Group
Following the baseline phase, participants (n = 249) in the control group were provided with research cigarettes that contained amounts of nicotine comparable to usual-brand cigarettes (15.8 mg/g nicotine) for the 20-week experimental phase.
All cigarettes were administered under double-blind conditions. Participants were asked to return their cigarettes at each lab visit, and then the next set of cigarettes was provided.
Research Cigarettes
Spectrum brand research cigarettes were used for all research conditions.18 All cigarettes were produced by 22nd Century Group and provided free of charge by the National Institute of Drug Abuse (NOT NOT-DA-14-004).
Measures
Demographics
At the initial session, participants were queried about their race, gender, and age.
Fagerström Test for Nicotine Dependence
At the initial session, participants were administered the 7-item Fagerström Test for Nicotine Dependence (FTND).19
Nicotine Metabolite Ratio
From saliva samples collected at baseline, the ratio of 3′-hydroxycotinine to cotinine was calculated as a measure of CYP2A6 metabolic activity, reflecting the rate of nicotine metabolism.20
Cigarettes per Day
The number of total (study plus nonstudy) cigarettes smoked each day was calculated from the IVR telephone reports.
Total Nicotine Equivalents
Participants provided first void urine samples that were analyzed for total nicotine equivalents (TNEs) at Week 20.
Cigarette Evaluation Scale
The Cigarette Evaluation Scale (CES) uses a 1- to 7-item Likert scale (not at all to extremely) to assess subjective effects of cigarettes.21 From the CES, five subscales were derived22: Psychological Reward, assessing rewarding effects such as calming you down and feeling more awake; Smoking Satisfaction, assessing whether smoking was satisfying and enjoyable; the single-item assessment of Enjoyment of Respiratory Tract Sensations; the single-item subscale assessing Craving Reduction; and the Aversion subscale assessing negative subjective evaluations including nausea and dizziness. The CES was administered at each laboratory visit.
During the baseline phase, all participants were asked to rate their responses to their usual brand cigarettes on the CES. During the experimental phase, participants were asked to rate their responses to their study cigarettes over the past 2 weeks on the CES. Thus, relevant to this analysis, at Week 20, participants in both the immediate and the gradual conditions were responding to their experiences with the 0.4 mg/g (VLNC) cigarettes, and participants in the control condition were responding to their experiences with the 15.5 mg/g cigarettes.
Statistical Analysis
Study sample characteristics (gender, race, baseline CPD, FTND, nicotine metabolite ratio, CES subscale values, and TNEs) from the full sample and outcome variables at baseline were summarized using descriptive statistics, and means for each variable were compared across young and older age groups using t-tests for continuous variables and chi-square tests for binary variables. To determine the differences between condition (immediate vs control, gradual vs control, gradual adults immediate) within each age group, linear regression was used with an interaction term between dichotomized age and study condition to assess moderation of treatment effects by age on CPD and TNEs, with significant mean differences tested for between the gradual reduction and control, immediate reduction and control, and immediate versus gradual reduction groups. TNE values were natural-log transformed to correct for skewness; CPD values were not transformed. We ran separate models for each of the outcome variables. All linear regression models included condition, race (White, African American, or other), gender, FTND score at baseline, nicotine metabolite ratio at baseline, and baseline level of the outcome (TNEs or CPD at baseline).
Moderation by age was indicated by a significant interaction between age and treatment group. Next, we tested the effects of age group within condition on each CES subscale score at Week 20. To determine the differences between age groups within each study condition (immediate, gradual, and control), linear regression was used with an interaction term between dichotomized age and each of the three study conditions to assess significant mean differences between the two age groups within each study condition. Again, we ran separate models for each of the outcome variables. A significant difference by age was indicated by a significant mean difference across age group within condition. All linear regression models included condition, race (White, African American, or other), gender, FTND score at baseline, nicotine metabolite ratio at baseline, and the corresponding CES subscale score at baseline (in relation to Usual Brand cigarettes).
Sensitivity Analyses
Previous research has shown that compliance with the study cigarettes may be different across age groups.23 To determine whether results were consistent when accounting for self-reported compliance, the models described above were rerun using only those participants who self-reported full compliance with the study cigarettes (ie, no nonstudy cigarettes reported) across all groups at each time point consistent with previous research.17 Due to a limited sample size of biochemically verified compliance in the young adult age group, self-report was used as measure of compliance.
All data were analyzed using Statistical Analysis System software version 9.4 (SAS Institute Inc., Cary, NC). The significance level for p-values was set at α = 0.05.
Results
Participant Characteristics
This intent-to-treat analysis included 1250 subjects. Of these, 958 completed all study procedures (N = 53 young adults [ages 18–24], N = 905 older adults [ages 25+]). Completion rates were statistically different across age group (p < .01) such that there were fewer young adult completers (61%) at Week 20 relative to older adults (79% completers). A detailed summary of the sample characteristics from the parent study can be found in the primary paper from the parent study.7 The relevant sample characteristics for this secondary analysis can be found in Table 1. Overall, the young adults were more likely to be male and white than their older counterparts. Furthermore, as is frequently found in this age group, at baseline young adults reported significantly lower dependence, smoked fewer CPD, and had lower levels of nicotine exposure as indicated by lower TNEs than the older adults. No differences by age group were found with respect to nicotine metabolite ratio. With respect to subjective responses to their usual brand cigarettes, young adults reported higher levels of Psychological Reward and Enjoyment of Respiratory Tract Sensations relative to older adults. No differences were found across age groups with respect to Smoking Satisfaction, Craving Reduction, or Aversion.
Table 1.
Baseline Characteristics by Age Group Category
| Characteristic | Age group | |||
|---|---|---|---|---|
| 18–24 | ≥25 | Effect sizea (95% CI) | p | |
| N | 87 | 1163 | ||
| Treatment group, number (%) | .21 | |||
| Gradual reduction | 27 (31.0) | 571 (40.5) | ||
| Immediate reduction | 41 (47.1) | 462 (39.7) | ||
| Control | 19 (21.8) | 230 (19.8) | ||
| Age in years, mean (SD) | 21.9 (1.8) | 46.8 (12.2) | ||
| Gender, number, % male | 58 (66.7) | 643 (55.3) | .04 | |
| Race, number (%) | <.01 | |||
| White | 63 (72.4) | 695 (59.8) | ||
| Black | 8 (9.2) | 365 (31.4) | ||
| Other | 14 (16.1) | 82 (7.1) | ||
| Cigarettes per day | 14.8 ± 6.8 | 17.3 ± 8.6 | 0.3 (0.1, 0.5) | <.01 |
| FTND, mean (SD) | 3.7 ± 1.7 | 4.3 ± 1.7 | 0.3 (0.1, 0.5) | <.01 |
| Psychological Reward at baseline, mean (SD) | 3.6 ± 1.3 | 3.1 ± 1.4 | −0.4 (−0.6, −0.2) | <.01 |
| Smoking Satisfaction at baseline, mean (SD) | 4.8 ± 1.4 | 4.7 ± 1.4 | −0.1 (−0.3, 0.1) | .39 |
| Enjoyment of Respiratory Tract Sensations at baseline, mean (SD) | 4.1 ± 1.7 | 3.4 ± 1.7 | −0.4 (−0.6, −0.2) | <.01 |
| Craving Reduction at baseline, mean (SD) | 4.3 ± 1.9 | 4.5 ± 1.9 | 0.1 (−0.1, 0.4) | .24 |
| Aversion, mean (SD) | 1.3 ± 0.5 | 1.3 ± 0.6 | 0.0 (−0.2, 0.2) | .97 |
| TNEs at baseline, geometric mean (range) | 44.7 (3.7–148.4) | 60.3 (0.2–492.7) | 0.4 (0.2, 0.6)b | <.01 |
| Nicotine metabolite ratio at baseline, mean (SD) | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.1 (−0.1, 0.3) | .42 |
p-Values represent t-tests of differences for continuous variables and chi-square tests for categorical variables. Subjective response scores were in reference to participants’ usual brand cigarettes at baseline. Bold values indicate significant p-value. p-value for TNEs is derived from a t-test comparing log-transformed TNE values. CI = confidence interval; FTND = Fagerström Test for Nicotine Dependence; TNE = total nicotine equivalents.
aEffect size = difference in means (older age group − younger age group) by pooled standard deviation.
bEffect size reported was on log transformed TNEs.
Moderation of Treatment Effects on Primary Outcomes (TNEs and CPD) by Age Group
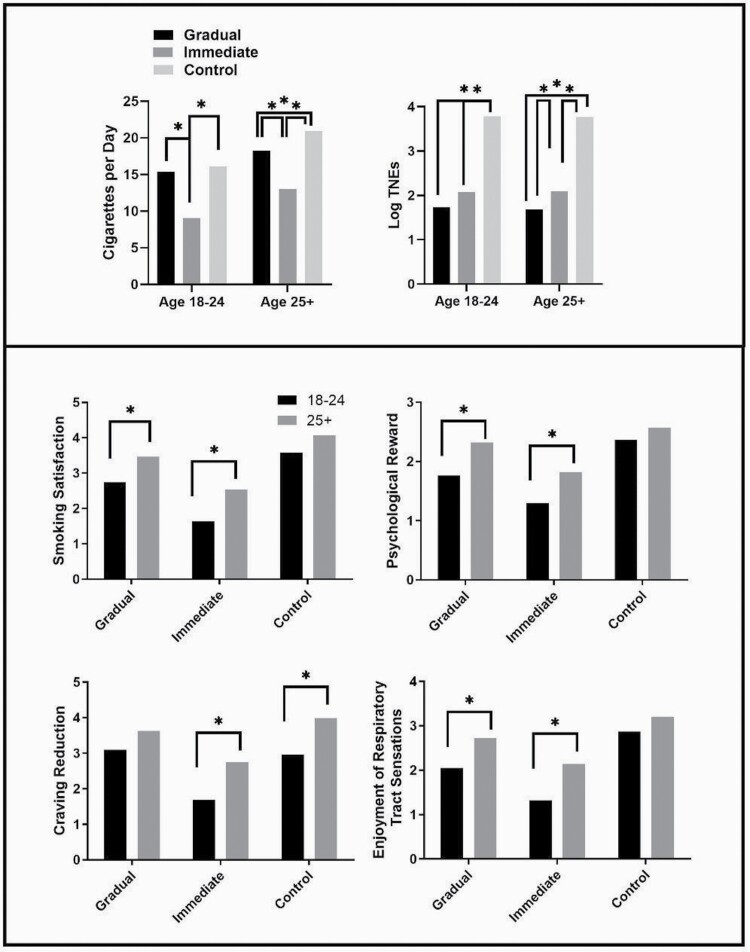
Table 2 presents the results for primary outcomes collected at Week 20. At Week 20, young adults smoked 9.0 CPD in the immediate condition, whereas older adults smoked 13.0 CPD on average. In the gradual condition, young adults smoked 15.3 CPD, whereas older adults smoked 18.2. In the control condition, young adults smoked 16.0 CPD, whereas older adults smoked 20.9. These results are depicted in the top panel of Figure 1, along with the results of the TNE comparisons. Overall, the treatment effects were consistent across age groups, and the interactions were not significant for either end point. In the 18–24 age group, the immediate condition resulted in significantly lower mean levels of both TNEs and CPD relative to control, whereas the gradual condition resulted in a significantly lower mean level of TNEs only. In the 25+ age group, both the immediate and gradual conditions resulted in significantly lower mean levels of both TNEs and CPD relative to control, although the decrease in CPD was substantially larger in the immediate condition. When comparing the effects of the immediate condition to the gradual condition, the immediate condition led to significantly lower CPD than the gradual condition for both younger and older adults, whereas the immediate condition led to significantly lower TNEs relative to the gradual condition in the older adults, but not in the young adults.
Table 2.
Mean and Standard Errors of Least-Squares Mean Differences at Week 20 Between Treatment Groups as a Function of Age Group (Top Panel, Age 18–24 and Bottom Panel, Age ≥ 25)
| Age 18–24 | |||||||
|---|---|---|---|---|---|---|---|
| Outcome | Interaction p-value | Immediate vs control | p | Immediate vs gradual | p | Gradual vs control | p |
| Total CPD | .80 | −7.02 (2.98) | 0.02 | −6.32 (2.84) | 0.03 | −0.70 (2.97) | .82 |
| Log TNE | .99 | −1.71 (0.57) | <0.01 | 0.35 (0.51) | 0.50 | −2.05 (0.57) | <.01 |
| Age ≥ 25 | |||||||
| Outcome | Interaction p-value | Immediate vs control | p | Immediate vs gradual | p | Gradual vs control | p |
| Total CPD | .80 | −7.95 (0.78) | <.01 | −5.20 (0.65) | <.01 | −2.74 (0.76) | <.01 |
| Log TNE | .99 | −1.68 (0.15) | <.01 | 0.40 (0.13) | <.01 | −2.08 (0.15) | <.01 |
Interaction test p-values represent the outcome of tests for overall significant interactions between age category and nicotine reduction group, and p-values listed for each comparison shows the significance of least-squares mean differences between age groups within each nicotine reduction group. Mean difference p-values represent the outcome of post hoc contrast test probing the interaction for significant differences by age group. Positive mean difference values indicate higher values in the younger adults compared with older adults. Regression model adjusted for age group (18–24, ≥25), nicotine group (gradual, immediate, control), value of the given outcome at baseline, FTND at baseline, race (White, African American, other), nicotine metabolite ratio at baseline and gender. Bold values indicate significant p-value. CPD = cigarettes per day, TNEs = total nicotine equivalents.
Figure 1.
Mean predicted values from all outcome variables with any significant differences at Week 20. The top panel shows treatment effects across age groups on primary outcomes; the lower panel shows comparisons within condition across age groups on subjective effects. Asterisks represent a significant mean difference.
Subjective Responses to Study Cigarettes Within Condition by Age Group
Table 3 presents the results for subjective outcomes collected at Week 20. These results are depicted in the bottom panel of Figure 1. Overall, the effects were consistent across age groups and the interactions were not significant. Within the gradual condition, relative to older adults, young adults’ evaluations of VLNC cigarettes were lower on the CES Smoking Satisfaction, Psychological Reward, and Enjoyment of Respiratory Tract Sensations factors, but not on the Craving Reduction or Aversion factors. In the immediate condition, relative to older adults, young adults’ evaluations of VLNC cigarettes were lower on CES Smoking Satisfaction, Psychological Reward, Enjoyment of Respiratory Tract Sensations, and Craving Reduction, but not on Aversion. In the control condition, younger adults’ evaluations of normal nicotine content cigarettes on the Craving Reduction scale were significantly lower than those of older adults; no other differences across age group on subjective outcomes were found in the control condition.
Table 3.
Means and Standard Errors of Least-Squares Mean Differences Week 20 Between Age Groups (Age 18–24 and Age ≥25) as a Function of Treatment Group
| Outcome | Interaction test p-value | Immediate reduction | Gradual reduction | Control | |||
|---|---|---|---|---|---|---|---|
| Mean difference | p | Mean difference | p | Mean difference | p | ||
| CES SSa | .71 | −0.91 (0.31) | <.01 | −0.71 (0.31) | .02 | −0.49 (0.39) | .21 |
| CES PR a | .60 | −0.52 (0.23) | .03 | −0.56 (0.23) | .02 | −0.20 (0.29) | .49 |
| CES ERTSa | .62 | −0.82 (0.32) | .01 | −0.67 (0.32) | .03 | 0.33 (0.40) | .41 |
| CES CRa | .61 | −1.07 (0.41) | .01 | −0.54 (0.41) | .18 | −1.03 (0.51) | .04 |
| CES AVa | .48 | −0.01 (0.17) | .93 | −0.05 (0.17) | .76 | −0.32 (0.21) | .12 |
| Total CPDa | .69 | −3.92 (0.06) | .06 | −2.82 (1.21) | .17 | −4.88 (2.30) | .03 |
| Log TNEsa | .98 | −0.12 (0.36) | .74 | −0.04 (0.37) | .92 | −0.00 (0.45) | 1.00 |
Interaction test p-values represent the outcome of tests for overall significant interactions between age category and nicotine reduction group and mean differences between age groups at visit 20. Mean difference p-values represent the outcome of post hoc contrast test probing the interaction for significant differences by nicotine group. Positive mean differences values indicate higher values in the younger adults compared with older adults. Regression model adjusted for age group (18–24, ≥25), nicotine content group, value of the given outcome at baseline, FTND at baseline, race (White, African American, other), nicotine metabolite ratio at baseline, and gender. Bold values indicate significant p-value at α = 0.05. CES = Cigarette Evaluation Scale; SS = Smoking Satisfaction, PR = Psychological Reward; ERTS = Enjoyment of Respiratory Tract Sensations; CR = Craving Reduction; AV = Aversion; CPD = cigarettes per day; TNEs = total nicotine equivalents.
Sensitivity Analyses
In this sample, no significant differences were found in terms of self-reported compliance across age groups (all p’s > .10). Among participants who self-reported total compliance with the study cigarettes at Week 20 (N = 41 young adults, N = 746 older adults), the pattern of results remained generally the same as for the full sample: young adults smoked fewer study CPD than older adults at Week 20 in the immediate group, but this difference was not evident in the gradual or control groups, and TNEs were not significantly different across age group. In terms of subjective outcomes, young adults showed significantly less smoking satisfaction, craving reduction, and enjoyment of respiratory symptoms relative to older adults in the immediate group, but these differences were not evident in the gradual or control groups. Aversion was not different across age groups.
Discussion
The current study demonstrated that for both young and older adults, the strategy of immediately switching participants to VLNC cigarettes resulted in lower CPD and TNEs at Week 20 than the control condition. The gradual nicotine reduction strategy also led to lower CPD and TNEs compared with the control condition among the older adults, but only TNEs were lower among younger adults. When comparing the two nicotine reduction conditions directly, immediate reduction resulted in lower CPD for both young and older adults; however, there were no differences across the two conditions for TNEs in young adults. It is also important to note that relative to control, after 20 weeks, the magnitude of reduction in CPD was larger in the immediate condition relative to the gradual condition for both young and older adults, potentially due to longer exposure to VLNC cigarettes. As an immediate reduction approach was more effective for reducing biomarkers of tobacco exposure,7 it is encouraging that this approach led to overall lower levels of smoking in this sample of young adults after 20 weeks of exposure and that treatment effects were consistent across age groups.
We also found that young adults responded differently than older adults in terms of subjective response to VLNC cigarettes, such that within each condition, young adults reported lower positive subjective responses to cigarettes regardless of whether they had been introduced to them immediately or gradually. These results replicate and expand upon our previous findings from a trial that tested the effects of immediate nicotine reduction,17 which indicated that young adults showed reduced positive subjective responses relative to older adults after 2 weeks of use. However, in our previous study, differences between older and younger adults had dissipated after 6 weeks of use across all outcomes, whereas in the current study, differences persisted through 20 weeks of use. One potential reason for the discrepancy could be that in the previous study, we combined results from three low nicotine content groups (2.4–0.4 mg) to increase statistical power, whereas in the current study, all participants in the immediate group were exposed to 0.4 mg/g only. It could be that responses to the slightly higher nicotine contents influenced the outcome in the previous study. As in our previous paper, no differences were found across younger and older adults for aversion to low nicotine cigarettes.
Finally, in line with our previous work, despite significant differences in cigarettes smoked per day, TNE data did not always appear to reflect smoking rate differences in the young adults in the sample. This indicates noncompliance with study cigarettes, which could include use of usual brand cigarettes and/or use of other nicotine-containing products such as e-cigarettes. It is important to note that when analyses included only those participants who self-reported full compliance with use of study cigarettes, the age difference in TNE did not emerge at Week 20; this indicates that participants may have under-reported noncompliance at Week 20.
Overall, the results of this study indicate that an immediate reduction in cigarette nicotine content would potentially result in less smoking in both young and older adults, as well as a potentially greater reduction in abuse liability in young adults, than a gradual reduction in cigarette nicotine content. It is important to underscore that in both reduction conditions, young adults rated VLNC cigarettes more negatively than did older adults, and young adults smoked fewer VLNC cigarettes than did older adults. This is key information because it is generally understood that youth and young adults smoke cigarettes for many reasons unrelated to nicotine, including due to peer influence, and because smoking fits with their identity.24–26 Thus, one might hypothesize that substantially reducing the nicotine from cigarettes may affect young adult smoking behavior less than older adults, which has not been borne out by this or our previous study.17
Smoking cigarettes is maintained not only by the primary reinforcing effects of nicotine administration, but also by the conditioned reinforcing value of the other sensorimotor stimuli (eg, smoke inhalation, taste, etc.) that have acquired reinforcing effects as a function of their history of being paired with nicotine administration; thus even without nicotine, VLNC cigarettes can alleviate craving.27–30 As conditioned reinforcing effects gain strength over time,31 young adults who have smoked for shorter periods of time may experience less conditioned reinforcing effects of cigarettes.15 In this case, one mechanism that may account for the lower satisfaction and use of VLNC cigarettes in younger adults relative to older adults is that younger adults may experience weaker conditioned reinforcing effects of VLNC cigarette use. Such a mechanism would explain why, when nicotine is reduced in cigarettes and a greater proportion of smoking reinforcement may be driven by conditioning, young people experience less reinforcement overall. This hypothesis requires further study, and other hypotheses are also plausible. Adolescent brain development differs greatly from adults, and nicotine is known to differentially affect youth, both in terms of neurochemistry and acquisition of nicotine reinforcement32–34; as the brain continues to develop up through young adulthood,35 the loss of nicotine from cigarettes may affect young adults to a greater extent than adults such that VLNC cigarettes are even less satisfying. Future work can elucidate which mechanisms are most heavily involved in driving differences in satisfaction between younger and older adults.
The strengths of this study include the relatively long exposure period of 20 weeks and the large overall sample size. However, limitations of these data exist. First, the gradual and immediate groups differed not only on their method of reaching the lowest content but also on their duration of exposure to the lowest nicotine content, which cannot be fully disentangled in the current study design. However, secondary analyses from the parent study have shown that participants in the immediate group at Week 4 showed lower satisfaction relative to the gradual group at the Week 20 (ie, after the gradual group had an equal 4 weeks of VLNC exposure); indicating that this may be due to less to the greater duration of exposure rather than to the method of reduction per se.7,36 Second, the sample, while relatively large overall, included relatively few young adults relative to older adults, and these unequal samples could have affected the results by misestimating the true results of such a policy in young adults. Furthermore, the young adults in this sample were relatively heavy smokers compared with the broader population of young adults who smoke due to the inclusion criteria, which stipulated smoking at least 5 CPD. Many young adults are intermittent or light daily smokers,37,38 and therefore, results from these participants may not fully generalize to all young adult smokers. Although research with intermittent-smoking older adults has shown that VLNC cigarettes reduce smoking,39 future research should focus on effects of VLNC cigarettes in intermittent or light daily smoking young adults. In addition, this study excluded those who frequently use other nicotine-containing products such as e-cigarettes and little cigars, which is common in young adults10,40; thus, more work remains to be done to determine how effects may generalize to polytobacco-using youth. However, although the young adults in this study may not be representative of all young adult smokers, they do represent a high-risk group of young adults who are on a heavier smoking trajectory. Despite these limitations, results from the current study and our previous study17 consistently indicate that substantially reducing the nicotine content of cigarettes leads to reductions in use in both young and older adults, and greater reductions in cigarette satisfaction in younger adults relative to older adults. Furthermore, results from this study indicate that reducing the nicotine content of cigarettes immediately rather than gradually may lead to the most potent health gains among this high-risk group of young adult daily smokers.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Funding
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. Research supported by a grant from the National Institute on Drug Abuse and the Food and Drug Administration Center for Tobacco Products (U54DA031659; Donny/Hatsukami). Manuscript preparation supported by NCI K01CA189300 (PI Cassidy), K01DA047433 (Smith), and NIDA P50DA036114.
Declaration of Interests
None declared.
References
- 1.USDHHS. The Health Consequences of Smoking – 50 Years of Progress: A Report of the Surgeon General. Rockville, MD: Public Health Service, Office on Public Health; 2014. [Google Scholar]
- 2.Jha P, Ramasundarahettige C, Landsman V, et al. . 21st-Century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–350. doi: 10.1056/NEJMsa1211128 [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb S, Zeller M. A nicotine-focused framework for public health. N Engl J Med. 2017;377(12):1111–1114. doi: 10.1056/NEJMp1707409p [DOI] [PubMed] [Google Scholar]
- 4.Apelberg BJ, Feirman SP, Salazar E, et al. . Potential public health effects of reducing nicotine levels in Cigarettes in the United States. N Engl J Med. 2018;378(18):1725–1733. doi: 10.1056/NEJMsr1714617 [DOI] [PubMed] [Google Scholar]
- 5.Donny EC, Denlinger RL, Tidey JW, et al. . Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. doi: 10.1056/NEJMsa1502403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benowitz NL, Dains KM, Hall SM, et al. . Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21(5):761–769. doi: 10.1158/1055-9965.EPI-11-0644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatsukami DK, Luo X, Jensen JA, et al. . Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure. JAMA. 2018;320(9):880. doi: 10.1001/jama.2018.11473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YO, Hebert CJ, Nonnemaker JM, Kim AE. Youth tobacco product use in the United States. Pediatrics. 2015;135(3):409–415. doi: 10.1542/peds.2014-3202 [DOI] [PubMed] [Google Scholar]
- 9.Colby SM, Tiffany ST, Shiffman S, Niaura RS. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug Alcohol Depend. 2000;59 (suppl 1):S83–S95. http://www.ncbi.nlm.nih.gov/pubmed/10773439. Accessed December 1, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Kasza KA, Ambrose BK, Conway KP, et al. . Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med. 2017;376(4):342–353. doi: 10.1056/NEJMsa1607538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoeppner BB, Bidwell LC, Colby SM, Barnett NP. Smoking patterns and their relationship to drinking among first-year college students. Nicotine Tob Res. 2014;16(6):743–752. doi: 10.1093/ntr/ntt205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Ryzin MJ, Fosco GM, Dishion TJ. Family and peer predictors of substance use from early adolescence to early adulthood: an 11-year prospective analysis. Addict Behav. 2012;37(12):1314–1324. doi: 10.1016/j.addbeh.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews JA, Tildesley E, Hops H, Li F. The influence of peers on young adult substance use. Health Psychol. 2002;21(4):349–357. http://www.ncbi.nlm.nih.gov/pubmed/12090677. Accessed March 21, 2018. [DOI] [PubMed] [Google Scholar]
- 14.O’Loughlin J, O’Loughlin EK, Wellman RJ, et al. . Predictors of cigarette smoking initiation in early, middle, and late adolescence. J Adolesc Heal. 2017;61(3):363–370. doi: 10.1016/j.jadohealth.2016.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenter MJ, Saladin ME, Larowe SD, et al. . Craving, cue reactivity, and stimulus control among early-stage young smokers: effects of smoking intensity and gender. Nicotine Tob Res. 2014;16(2):208–215. doi: 10.1093/ntr/ntt147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtin JJ, Barnett NP, Colby SM, Rohsenow DJ, Monti PM. Cue reactivity in adolescents: measurement of separate approach and avoidance reactions. J Stud Alcohol. 2005;66(3):332–343. http://www.ncbi.nlm.nih.gov/pubmed/16047522. Accessed September 27, 2016. [DOI] [PubMed] [Google Scholar]
- 17.Cassidy RN, Tidey JW, Cao Q, et al. . Age moderates smokers’ subjective response to very-low nicotine content cigarettes: evidence from a randomized controlled trial. Nicotine Tob Res. 2019;21(7):962–969. doi: 10.1093/ntr/nty079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richter P, Pappas RS, Bravo R, et al. . Characterization of SPECTRUM variable nicotine research cigarettes. Tob Regul Sci. 2016;2(2):94–105. doi: 10.18001/TRS.2.2.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3(3–4):235–241. http://www.ncbi.nlm.nih.gov/pubmed/735910. Accessed December 1, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. doi: 10.1124/pr.57.1.3 [DOI] [PubMed] [Google Scholar]
- 21.Arger CA, Heil SH, Sigmon SC, et al. . Preliminary validity of the modified Cigarette Evaluation Questionnaire in predicting the reinforcing effects of cigarettes that vary in nicotine content. Exp Clin Psychopharmacol. 2017;25(6):473–478. doi: 10.1037/pha0000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. doi: 10.1016/j.addbeh.2006.06.028 [DOI] [PubMed] [Google Scholar]
- 23.Nardone N, Donny EC, Hatsukami DK, et al. . Estimations and predictors of non-compliance in switchers to reduced nicotine content cigarettes. Addiction. 2016;111(12):2208–2216. doi: 10.1111/add.13519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran S, Wechsler H, Rigotti NA. Social smoking among US college students. Pediatrics. 2004;114(4):1028–1034. doi: 10.1542/peds.2003-0558-L [DOI] [PubMed] [Google Scholar]
- 25.Simons-Morton BG, Farhat T. Recent findings on peer group influences on adolescent smoking. J Prim Prev. 2010;31(4):191–208. doi: 10.1007/s10935-010-0220-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith DM, O’Connor RJ, Collins RL, Hyland AJ, Kozlowski LT. Correlates of smoker identity among intermittent and light daily young adult smokers: findings from Wave 1 of the Population Assessment of Tobacco and Health (PATH) Study. Addict Behav. 2019;98:106034. doi: 10.1016/j.addbeh.2019.106034 [DOI] [PubMed] [Google Scholar]
- 27.Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl). 2006;184(3–4):274–285. doi: 10.1007/s00213-005-0250-x [DOI] [PubMed] [Google Scholar]
- 28.Donny EC, Jones M. Prolonged exposure to denicotinized cigarettes with or without transdermal nicotine. Drug Alcohol Depend. 2009;104(1–2):23–33. doi: 10.1016/j.drugalcdep.2009.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dallery J, Houtsmuller EJ, Pickworth WB, Stitzer ML. Effects of cigarette nicotine content and smoking pace on subsequent craving and smoking. Psychopharmacology (Berl). 2003;165(2):172–180. doi: 10.1007/s00213-002-1242-8 [DOI] [PubMed] [Google Scholar]
- 30.Cassidy RN, Colby SM, Tidey JW, et al. . Adolescent smokers’ response to reducing the nicotine content of cigarettes: acute effects on withdrawal symptoms and subjective evaluations. Drug Alcohol Depend. 2018;188:153–160. doi: 10.1016/j.drugalcdep.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelleher RT, Gollub LR. A review of positive conditioned reinforcement. J Exp Anal Behav. 1962;5(4 suppl):543–597. doi: 10.1901/jeab.1962.5-s543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lydon DM, Wilson SJ, Child A, Geier CF. Adolescent brain maturation and smoking: what we know and where we’re headed. Neurosci Biobehav Rev. 2014;45:323–342. doi: 10.1016/j.neubiorev.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan M, Cross SJ, Loughlin SE, Leslie FM. N icotine and the adolescent brain. J Physiol. 2015;593(16):3397–3412. doi: 10.1113/JP270492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schassburger RL, Pitzer EM, Smith TT, et al. . Adolescent rats self-administer less nicotine than adults at low doses. Nicotine Tob Res. 2016;18(9):1861–1868. doi: 10.1093/ntr/ntw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson SB, Blum RW, Giedd JN. Adolescent maturity and the brain: the promise and pitfalls of neuroscience research in adolescent health policy. J Adolesc Heal. 2009;45(3):216–221. doi: 10.1016/j.jadohealth.2009.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith TT, Donny EC, Luo X, et al. . The impact of gradual and immediate nicotine reduction on subjective cigarette ratings. Nicotine Tob Res. 2019;21(suppl 1):S73–S80. doi: 10.1093/ntr/ntz158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hair E, Bennett M, Williams V, et al. . Progression to established patterns of cigarette smoking among young adults. Drug Alcohol Depend. 2017;177:77–83. doi: 10.1016/j.drugalcdep.2017.03.040 [DOI] [PubMed] [Google Scholar]
- 38.Pacek LR, Rass O, Sweitzer MM, Oliver JA, McClernon FJ. Young adult dual combusted cigarette and e-cigarette users’ anticipated responses to hypothetical e-cigarette market restrictions. Subst Use Misuse. 2019;54(12):2033–2042. doi: 10.1080/10826084.2019.1626435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiffman S, Kurland BF, Scholl SM, Mao JM. Nondaily smokers’ changes in cigarette consumption with very low-nicotine-content cigarettes: a randomized double-blind clinical trial. JAMA psychiatry. 2018;75(10):995–1002. doi: 10.1001/jamapsychiatry.2018.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rath JM, Villanti AC, Abrams DB, Vallone DM. Patterns of tobacco use and dual use in US young adults: the missing link between youth prevention and adult cessation. J Environ Public Health. 2012;2012:1–9. doi: 10.1155/2012/679134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.