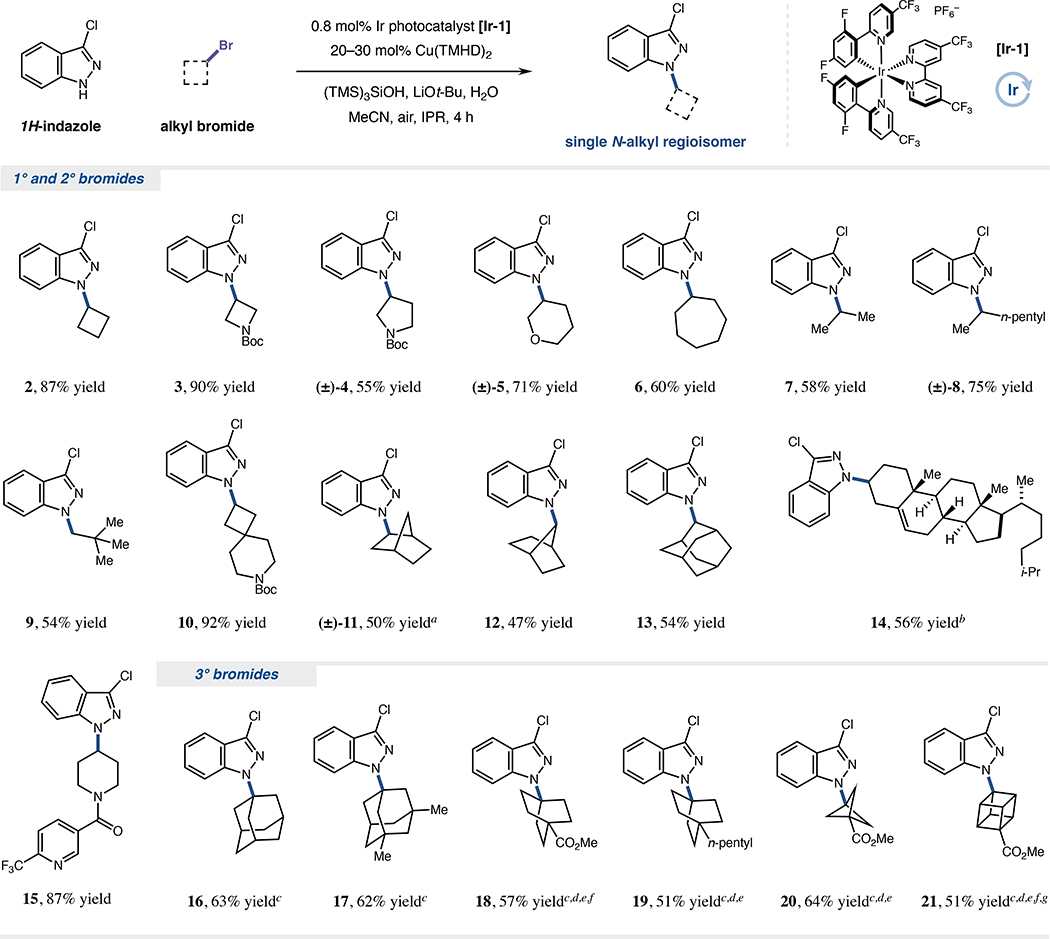
Figure 2. Alkyl bromide scope for copper-HARC N-alkylation.
Isolated yields unless otherwise indicated. r.r. >20:1 in all cases. Reactions generally performed under air with photocatalyst [Ir-1] (0.8 mol%), Cu(TMHD)2 (20–30 mol%), (TMS)3SiOH (2.5 equiv), LiOt-Bu (3.0 equiv), H2O (10 equiv), N-nucleophile (0.25 mmol, 1.0 equiv) and alkyl bromide (2.5 equiv) in MeCN (0.1 M) under IPR irradiation (450 nm) for 4 h. See Supplemental Information for specific experimental details. ad.r. 4:1. b0.05 mmol scale; toluene/MeCN (7:3, 0.1 M) as solvent; d.r. 1:1; yield determined by 1H NMR analysis. c(TMS)3SiNHAd as silyl radical source. d60 mol% Cu(TMHD)2. eIsolated yield from five combined 0.05 mmol scale reactions (0.25 mmol total) due to reduced yields (>5% loss of yield) on typical 0.25 mmol scale. fIr[dF(CF3)ppy]2(dtbbpy)PF6 [Ir-2] as photocatalyst. g3.5 equiv. (TMS)3SiNHAd used. Ad, 1-adamantyl.