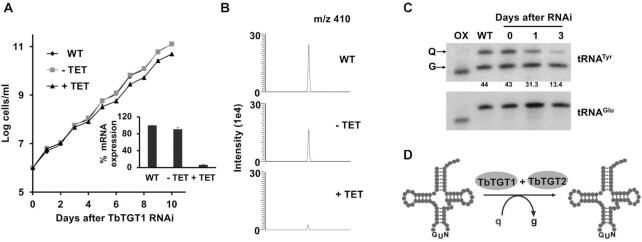
Figure 1.
TbTGT1 is necessary for Q formation in the tRNAs. (A) Growth curve of T. brucei procyclic form (PF) cells where TbTGT1 expression has been silenced by RNAi (+TET) compared to WT cells or an uninduced control (−TET). The data represents mean ± SD from three independent experiments. RNAi knock-downs were confirmed by qRT PCR (inset) and presented as % relative TGT1 mRNA expression, normalized to WT; (B) Extracted ion chromatogram for Q 410 m/z comparing samples from WT, TbTGT1 RNAi induced (+TET) and uninduced (−TET). Peak corresponding to m/z of 410 is 7 times reduced in +TET sample than –TET control. (C) APB gel - Northern hybridization showing the effect of RNAi knock-down of TbTGT1 in T. brucei on the levels of queuosine of tRNATyr (Numbers under the blot indicate %Q levels, calculated by dividing the intensity of the Q band by the sum of the Q and G bands, and multiplied by 100). tRNAGlu was used as a loading control; Oxidized RNA was used as a negative control (OX). 3-Aminophenylboronic acid (APB) has affinity for the cis-diol groups present in queuosine. As a result, Q-tRNAs migrate slower on the gel, leading to separation of modified (Q) and unmodified (G) tRNAs. In oxidized control (OX), the tRNAs are treated with periodate, to oxidize all cis-diol groups (queuosine as well as terminal 3′ ribose). (D) Schematic representation of enzymatic substitution of guanine (g) with queuine (q) by TbTGT heteromer.