Figure 8.
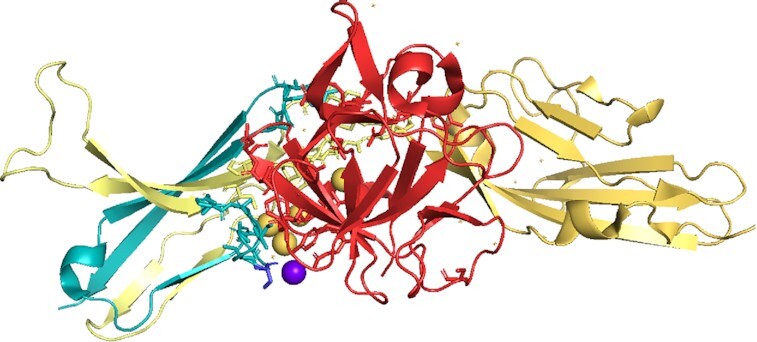
The tandem exon duplication-derived substitution in FGFR2 determines FGF binding specificity Ligand FGF10 bound to isoform FGFR2b in the PDB structure 1nun. FGF10 is in red, the constant part of FGFR2b in yellow and the tandem duplicated exon that generates half of domain 3 in teal. Residues involved in the interaction between receptor and ligand are shown in stick form. Waters that form part of hydrogen bonds are shown as spheres. Residue S320 (shown as a blue stick) has been associated with lung squamous cell cancer (71). The residue is very close to the binding site with FGF10. It is possible that the mutation to cysteine might allow it to form a hydrogen bond with a glutamate in FGF10 via a water molecule (purple sphere), thus strengthening the binding between FGFR2b and FGF10.
