Figure 4.
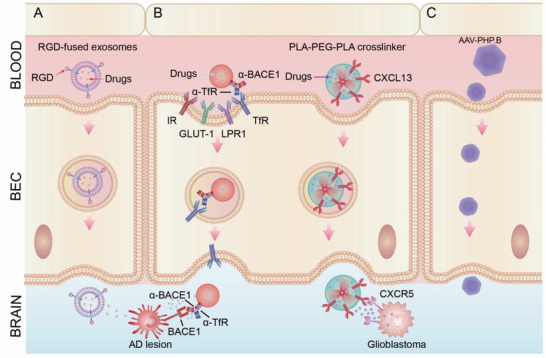
Employment of transcytosis for brain‐targeted delivery. A) Exosome‐based delivery. Exosomes that express a fusion protein of rabies virus glycoprotein (RVG) peptide, a short (29 amino acid) peptide derived from the RVG, could cross the BBB and specifically target brain cells. Systemic administration of RVG exosomes that carried siRNA against BACE1 substantially reduced levels of BACE1 mRNA expression in the brain, leading to better prognosis of AD. B) Nanoparticle‐based delivery. Engineering nanoparticles with multi‐functionalities can significantly improve the specificity and efficacy for targeted delivery. One example is a bispecific antibody, which is designed to recognize two different epitopes or antigens. A bispecific antibody targeting transferrin receptor (TfR) and BACE1 achieved superior efficacy in the treatment of AD. In the treatment of tumor in brain, Rituximab (RTX) is encapsulated within a biodegradable, crosslinked zwitterionic polymer to increase brain uptake. Further conjugating this nanocapsule with CXCL13 guided RTX nanoparticles to brain metastases of primary lymphoma to enhance the therapeutic efficacy of the antibody. C) Adeno‐associated viral (AAV) vectors are a rapidly emerging delivery platform for delivering gene and antibody‐based drugs to various cells, including neurons, astrocytes, and oligodendrocytes in the CNS and demonstrate encouraging safety and efficacy in clinical studies. AAVs enter cells via macropinocytosis, phagocytosis, clathrin, and caveolae‐mediated endocytosis. Engineered AAV capsids, AAV‐PHP.B, were recently reported to have unprecedented ability to transfer genes to the CNS in the adult mouse after systemic administration, with a >40‐fold enhancement over the previous standard AAV9, potentially transforming the ability to treat neurodegenerative diseases with AAV gene therapy.
