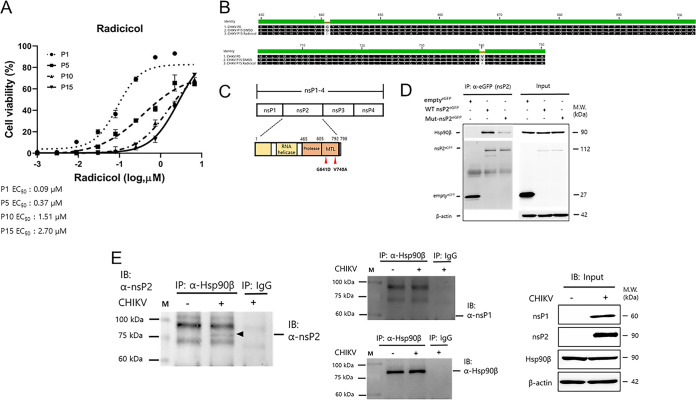
FIG 5.
Radicicol targets the MT-like domain of CHIKV nsP2, which interacts with Hsp90β. (A) The antiviral activity of radicicol against resistant virus strains selected by serial passages was determined. The dose-response curve (DRC) analysis was performed using Vero76 cells infected with radicicol-resistant variants from different passages in the presence of various concentrations of radicicol. At 48 hpi, cell viability was measured using the CellTiter 96 AQueous One Solution cell proliferation assay. (B) Amino acid sequence alignment of radicicol-resistant virus (CHIKV P15 radicicol). The residues substituted in the radicicol-resistant virus (G641D and V740A) are shown. (C) Schematic of the organization of the CHIKV genome encoding nsP1-4 (top) and the nsP2 protein. The residue substitutions are indicated. (D) Immunoprecipitation assay of GFP-tagged wild-type nsP2 (WT nsP2GFP) or GFP-tagged mutant (mut-nsP2GFP) nsP2 with Hsp90β. Briefly, GFP only (emptyGFP), WT nsPGFP, or mut-nsP2GFP were expressed in HEK-293T cells for 24 h. At 24 h posttransfection, cell lysates were subjected to coimmunoprecipitation using 2 μg of anti-GFP antibodies. Western blot analysis was performed to determine the interaction between CHIKV nsPs and Hsp90β. Isotype control IgG was used as a negative control to pull down the protein complex. (E) Immunoprecipitation assay of Hsp90β with CHIKV nsP1 or CHIKV nsP2 using virus-infected cell lysates. Briefly, HEK293T cells were mock infected or infected with CHIKV at an MOI of 2. At 24 h postinfection, cell lysates were subjected to coimmunoprecipitation using 10 μg of Hsp90β antibodies. Western blot analysis was performed to determine the interaction between Hsp90β and CHIKV nsP1 and nsP2. Isotype control mouse IgG2a was used as a negative control to pull down the protein complex.