ABSTRACT
A newly developed cryptococcal antigen (CrAg) semiquantitative (SQ) lateral flow assay (LFA) provides a semiquantitative result in a rapid one-step test instead of performing serial dilutions to determine CrAg titer. We prospectively compared the diagnostic performance of the CrAgSQ assay (IMMY) with the CrAg LFA (IMMY) on cerebrospinal fluid (CSF) samples collected from persons with HIV-associated meningitis. The CrAgSQ grades (1+ to 5+) were compared with CrAg LFA titers and quantitative CSF fungal cultures. Among 87 participants screened for HIV-associated meningitis, 60 had cryptococcal meningitis (59 CrAg positive [CrAg+] by LFA and 1 false negative due to prozone with CrAg LFA titer of 1:1,310,000 and culture positivity), and 27 had no cryptococcal meningitis by CrAg LFA or culture. The CrAgSQ on CSF had 100% (60/60) sensitivity and 100% specificity (27/27). CSF CrAg titers ranged from 1:5 to 1:42 million. CrAgSQ grades of 1+, 2+, 3+, 4+, and 5+ corresponded to median CrAg LFA titers of 1:<10, 1:60, 1:7,680, 1:81,920, and 1:1,474,000, respectively. CSF CrAgSQ grades 3+ or higher were always CSF culture positive. Mortality at 14 days for those with low CrAgSQ grade (1+ to 3+) was 5% (1/22) versus 21% (8/38) with high CrAgSQ grades (4+ to 5+) (P = 0.084). The CrAgSQ demonstrates excellent diagnostic performance, maintaining both the sensitivity and specificity of the CrAg LFA, and counters false-negative prozone effects. The CrAgSQ assay reading is more complex but does provide useful clinical information about disease burden and probability of culture positivity in a single rapid diagnostic test.
KEYWORDS: semiquantitative assay, Cryptococcus, cryptococcal antigen, cryptococcal meningitis, HIV, semiquantitative lateral flow assay
INTRODUCTION
Cryptococcal disease is a leading cause of morbidity and mortality in sub-Saharan Africa, where an estimated 75% of global deaths occur (1, 2). Yet the index of clinical suspicion for invasive fungal infections often remains low (3). Cryptococcal antigen (CrAg), an indicator of disseminated cryptococcal infection, can be detected in both blood and cerebrospinal fluid (CSF) using a lateral flow assay (LFA), which is more sensitive and specific than latex agglutination (4). Therefore, CrAg testing is useful in the diagnosis of both cryptococcal antigenemia (disseminated disease before meningitis onset) and cryptococcal meningitis. Blood CrAg LFA titers of ≥1:160 (by the IMMY CrAg LFA assay) are increasingly predictive of developing meningitis or death (5–8). Further, baseline CSF CrAg titers ≥1:1,280 have been associated with increased 2- and 10-week mortality in first-episode cryptococcal meningitis (9). CrAg titers can be determined using a serial dilution technique with the CrAg LFA; however, this process rapidly consumes supplies (adding cost), requires technical expertise, and is often delayed relative to points of clinical decision-making. Given the important potential clinical utility of real-time CrAg titers, interest exists in a rapid, point-of-care, semiquantitative CrAg test.
The current reference standard for CrAg testing is the FDA-approved IMMY CrAg LFA (IMMY, Norman, OK, USA). The CrAg LFA is an inexpensive and rapid diagnostic test that demonstrates excellent performance with >99% sensitivity and >99% specificity for the diagnosis of cryptococcosis (4). Alternative assays have not performed as well according to previously published analyses and are not FDA approved (10–12). IMMY has now developed the CrAgSQ, an immunochromatographic LFA for the semiquantitative detection of CrAg in serum, plasma, whole blood, and CSF (11). The test uses a strip with three test lines yielding 5 possible grades of increasing positivity, representing higher concentrations of CrAg in the sample. While the CrAgSQ has been validated in serum (11), appearing promising as an inexpensive, rapid, semiquantitative CrAg LFA test, its diagnostic performance has not been characterized in CSF.
The goal of this diagnostic accuracy study was to assess the performance of the new semiquantitative IMMY CrAgSQ on CSF from HIV-infected persons with suspected meningitis compared to the FDA-approved CrAg LFA (IMMY) and culture as a composite reference standard.
MATERIALS AND METHODS
Study design.
From February to September 2019, we prospectively tested 87 CSF samples from patients with suspected HIV-associated meningitis at Kiruddu Hospital in Kampala, Uganda. CSF was collected in a sterile 10-ml conical tube during standard-of-care lumbar puncture from those being investigated for HIV-associated meningitis. Quantitative CSF cultures determined the number of Cryptococcus CFU per milliliter of CSF on all samples (13). The CrAg LFA and CrAgSQ assays were performed simultaneously in real time on fresh specimens per the manufacturer’s instructions. We determined CrAg LFA titers for every positive CrAg LFA independent of the CrAgSQ result. Titers were obtained using serial 2-fold dilutions until the CrAg LFA no longer indicated a qualitatively positive result.
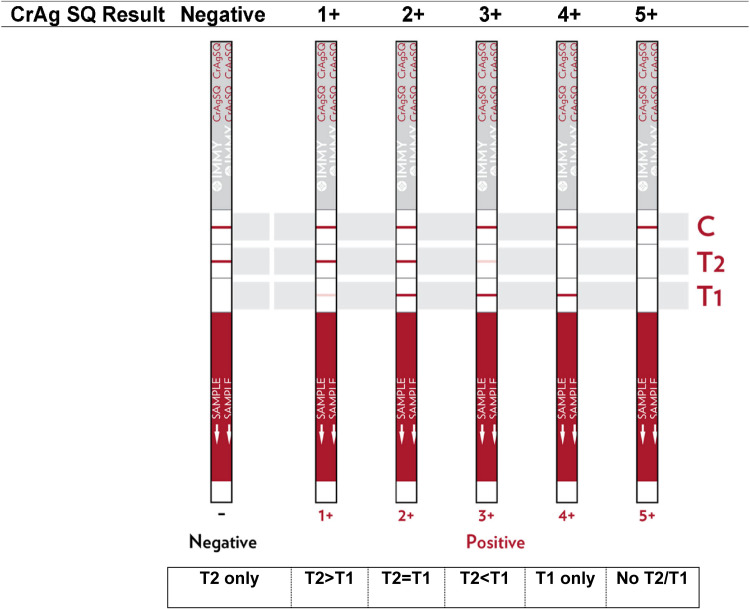
CrAgSQ testing is performed by adding 40 μl of CSF onto the “sample” end of the strip, followed by one drop of the supplied diluent. The strip is an immunochromatographic lateral flow assay that wicks the solution up the strip where gold-conjugated anti-CrAg antibodies and control antibodies bind with anchored antibodies in the strip, results in colorimetric change (visible line formation) at the binding sites. The CrAgSQ uses a three-line system on a wicking strip and results in negative, 1+, 2+, 3+, 4+, or 5+ grades. The assay uses an arrangement of test 1 (T1; sandwich formation), test 2 (T2; competitive inhibition), and control (C) lines, with T1 being the first line encountered by the wicking process and C being the last in a vertical orientation (Fig. 1). The semiquantitative grade read is made after 10 min. For our study, CrAgSQ grade interpretation was performed in real time by an experienced laboratory technician with manufacturer-supplied reference material available in a College of American Pathologists (CAP)-accredited laboratory at the Infectious Diseases Institute in Kampala, Uganda. The technician was blinded to the CrAg LFA result and future culture result.
FIG 1.
Semiquantitative CrAgSQ lateral flow assay visual interpretation guide. Visual interpretation guide provided with package insert in testing kit from IMMY [CrAgSQ package insert; IMMY], with simplified interpretation guide under the figure. Control (C) line will always be positive on a valid test. A greater than or lesser than sign refers to the relative line intensity on the strip. Biologic principles of assay adapted from IMMY CrAgSQ package insert as follows. “The test uses specimen wicking to capture gold-conjugated anti-CrAg monoclonal antibodies and gold-conjugated control antibodies deposited on the test membrane. If CrAg is present in the specimen, it binds to the gold-conjugated anti-CrAg antibodies. The gold-labeled antibody-antigen complex continues to wick up the membrane where it will interact with the two test lines (T1 and T2). The T1 line is a sandwich line, which contains immobilized anti-CrAg monoclonal antibodies. The gold-labeled antibody-antigen complex forms a sandwich at the test line causing a visible line to form. The T2 line is an inhibition (competitive) line, which contains immobilized cryptococcal antigen. Specimens that contain a high concentration of antigen, will inhibit the binding of the gold-conjugated anti-CrAg antibodies at the T2 line. With the proper flow and reagent reactivity, the wicking of any specimen, positive or negative, will cause the gold-conjugated control antibody to move to the control line (C). Immobilized antibodies at the control line will bind to the gold-conjugated control antibody and form a visible control line. Positive test results create either two (T1 and C) or three lines (T1, T2, and C). The presence of only a control line indicates an extremely high positive result. Negative test results will form two lines (T2 and C).”
All patients with cryptococcal meningitis were treated with amphotericin B (0.7 to 1.0 mg/kg/day) and fluconazole (1,200 mg/day) for a 14-day induction period, consistent with WHO standard of care. All participants in our studies additionally receive routine lab monitoring for drug-induced toxicities and supportive care with intravenous fluids and electrolyte repletion. Patients subsequently receive consolidation and maintenance therapies with fluconazole monotherapy.
Statistical analysis.
We analyzed data using SPSS version 25 (IBM, Armonk, New York, USA). We calculated the CrAgSQ sensitivity and specificity compared with a composite reference standard of CrAg LFA or culture positivity in those with HIV-associated meningitis to determine “true-positive” cryptococcal meningitis, while “true negatives” were determined by the CrAg LFA and culture resulting negative. McNemar’s test was used to assess for marginal homogeneity among the paired nominal data. CrAgSQ grades were plotted against LFA titers and CSF quantitative culture. Correlation coefficients for the ordinal-ranked CrAgSQ versus CrAg LFA titer or quantitative cryptococcal culture were calculated with Kendall’s tau. We assessed associations between CrAgSQ grade and 14-day mortality using a Kaplan-Meier survival curve with log-rank testing.
Ethics statement.
Informed consent was obtained for collection of clinical data, diagnostic testing for research purposes, and storage of specimen. Surrogate consent was obtained for those lacking the capacity to provide informed consent themselves. Institutional review board approval was obtained from both Uganda regulatory authorities and the University of Minnesota. CrAg LFA kits were purchased from IMMY, and the CrAgSQ kits were donated as an experimental assay not otherwise available for purchase. IMMY did not otherwise influence the study design, data analysis, or manuscript writing, nor did they provide any financial support.
RESULTS
The 87-person study cohort included 59 (68%) men with a mean age of 36 (±10) years and a median CD4 count of 32 cells/μl (interquartile range [IQR], 12 to 68). Refer to Table S1 in the supplemental material for demographic data by group. From the 87 CSF samples from participants with suspected meningitis, 60 (69%) were confirmed to have cryptococcal meningitis by CrAg LFA titer and culture. By CrAg LFA qualitative testing, 59 (68%) tested positive, and 28 (32%) tested negative for cryptococcal antigen. One negative CrAg LFA test was the result of the prozone effect, a known phenomenon that occurs with extremely high fungal burden (LFA titer of 1:1,310,000), which yielded a false-negative CrAg LFA result. This false-negative result had a positive culture (175,000 Cryptococcus CFU/ml). By CrAgSQ qualitative testing, 60 (69%) tested positive, and 27 (31%) tested negative using the CrAgSQ. Therefore, the CrAgSQ yielded a sensitivity of 100% (60/60; 95% confidence interval [CI], 0.94 to 1.0) and specificity of 100% (27/27; 95% CI, 0.88 to 1.0) (McNemar’s test, P = 0.99) compared to the composite reference standard (Table 1).
TABLE 1.
Contingency table for the CrAgSQ and CrAg LFA tests compared to the CrAg LFA reference composite (by qualitative LFA titer or CSF culture)
| Assay | Result | IMMY CrAg LFA (by qualitative titer) or CSF culture result |
|
|---|---|---|---|
| No. positive | No. negative | ||
| IMMY CrAgSQ | No. positive | 60 | 0 |
| No. negative | 0 | 27 | |
| IMMY CrAg LFA | No. positive | 59 | 0 |
| No. negative | 1a | 27 | |
One specimen was negative by qualitative CrAg LFA due to prozone effect but positive with a 1:2 dilution, having a final CrAg LFA titer of 1:1,310,000. This is a true-positive specimen.
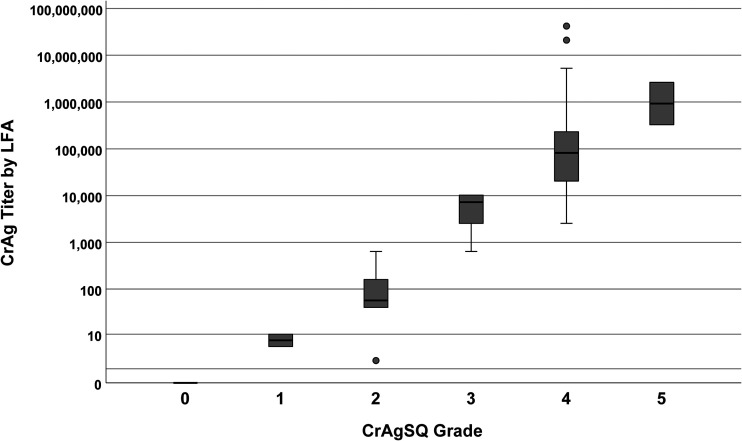
The CrAgSQ is designed to give a semiquantitative grade with each qualitatively positive result. Among the 60 persons with cryptococcal meningitis, the frequency of CrAgSQ grades were 1+ in 2 (3%), 2+ in 6 (10%), 3+ in 14 (23%), 4+ in 36 (60%), and 5+ in 2 (3%). We compared CSF CrAgSQ grades with LFA titers. CrAgSQ grades ranged from 1+ to 5+, while LFA titers ranged from 1:5 to 1: 41,943,040. A CrAgSQ grade of 1+ (n = 2) corresponded to a CrAg LFA range of 1:5 to 1:10. For grade 2+ (n = 6), the median LFA titer was 1:60 (IQR, 1:40 to 1:160; maximum, 1:640). For grade 3+ (n = 14), the median titer was 1:7,680 (IQR, 1:2,560 to 1:10,240; maximum, 1:10,240). For grade 4+ (n = 36), the median titer was 1:81,920 (IQR, 1:20,480 to 1:245,800; maximum, 1:41,943,040). For grade 5+ (n = 2), LFA titers were 1:327,700 and 1:2,621,000. CrAgSQ grades as relating to LFA titers are summarized in Fig. 2.
FIG 2.
Boxplot of CSF CrAg titer by LFA versus CrAgSQ grade. Boxplot of serial dilution CrAg titer for each CrAgSQ grade (1+ to 5+) is shown. Black bars represent median titer values, with the gray box borders representing the 25th (Q1) and 75th (Q3) percentiles (interquartile range). Whiskers represent minimum or maximum values, which are calculated as 1.5× Q1 or 1.5× Q3, respectively. Lone solid dots represent outliers. Increasing CrAgSQ grade trended positively with increasing CrAg LFA titer (Kendall’s tau-b [Τb] = 0.877, P < 0.001). Abbreviations: CSF, cerebrospinal fluid; CrAg, cryptococcal antigen; LFA, lateral flow assay.
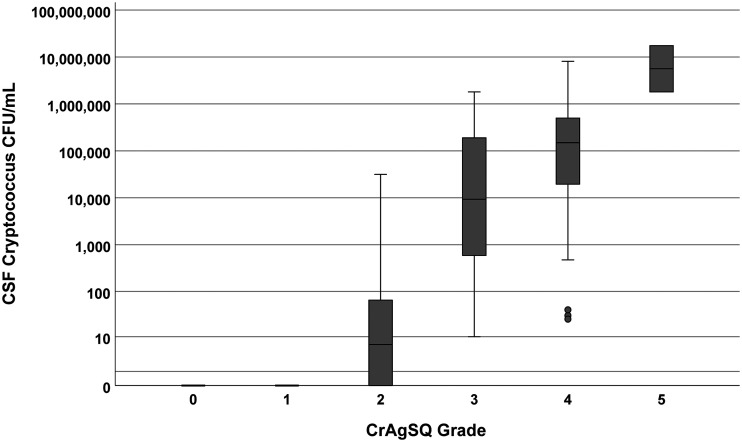
CSF CrAgSQ grades were also compared to fungal burden using the quantitative cryptococcal culture measurements. The two CrAgSQ 1+ grades had sterile cultures after 10 days of incubation. Of 6 CrAgSQ, grading of 2+ yielded a median of 28 CFU/ml (IQR, 0 to 65), with three cultures being sterile. For grade 3+, all cultures were positive with median growth of 10,700 CFU/ml (IQR, 590 to 190,000) and, for grade 4+, with median of 151,000 CFU/ml (IQR, 19,800 to 500,000). The two grade 5+ results averaged 9.63 million CFU/ml. Results are summarized in Fig. 3.
FIG 3.
Boxplot of quantitative Cryptococcus culture in CSF versus CrAgSQ grade. Boxplot of quantitative cryptococcal culture for each CrAgSQ grade (1+ to 5+) is shown. Black bars represent median titer values, with the gray box borders representing the 25th (Q1) and 75th (Q3) percentiles (interquartile range). Whiskers represent minimum or maximum values, which are calculated as 1.5× Q1 or 1.5× Q3, respectively. Lone solid dots represent outliers. Increasing CrAgSQ grade trended positively with increasing higher fungal burden in cerebrospinal fluid (Kendall’s tau-b [Τb] = 0.723, P < 0.001). Abbreviations: CSF, cerebrospinal fluid.
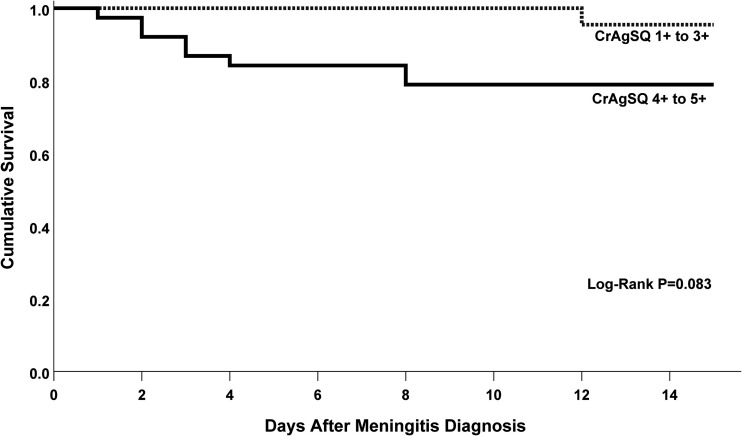
We examined the association of semiquantitative grade on 14-day survival. Overall 14-day mortality was 15% (9/60). No deaths occurred in eight participants with grades of 1+ or 2+. One death occurred with grade 3+ (7%; 1/14), seven deaths with grade 4+ (19%; 7/36), and one death with grade 5+ (50%; 1/2). Mortality at 14 days for those grouped with lower CrAgSQ grade (1+ to 3+) was 5% (1/22) versus 21% (8/38) with higher CrAgSQ grades (4+ to 5+). High CrAgSQ grade trended positively with higher mortality but was underpowered in a Kaplan-Meier model and did not reach statistical significance (P = 0.083) (Fig. 4).
FIG 4.
Kaplan-Meier Curve for survival at 14 days by CrAgSQ grade. Kaplan-Meier curve demonstrates a nonsignificant increased risk of death at 14 days in those with a high CrAgSQ grade (4+ to 5+) versus those with a low CrAgSQ grade (1+ to 3+).
DISCUSSION
The CrAgSQ has previously demonstrated good diagnostic performance on serum and plasma for the detection of disseminated cryptococcosis compared to the CrAg LFA, with sensitivity of 98% and specificity ranging from 95.8 to 97.6% (11, 14). However, CrAgSQ performance on CSF has not yet been published. Our prospective diagnostic accuracy study demonstrates excellent sensitivity (100%) and specificity (100%) of the IMMY CrAgSQ in detecting cryptococcal antigen in the CSF from persons with suspected HIV-associated meningitis.
An important advantage of the CrAgSQ over the LFA is its ability to detect the previously described prozone effect (15), as highlighted by a case in our study. The prozone effect occurs when an overwhelming burden of cryptococcal antigen prevents the binding of the preformed antibody-antigen complex to the strip-anchored antibody, preventing antibody-antigen-antibody sandwich formation. Without the sandwich formation anchored on the strip, no visible line appears, resulting in a false negative on the CrAg LFA. The CrAgSQ works around this by using a different binding modality at each line. In the case of potential prozone antigen burden, antibody-antigen-antibody sandwich formation is still blocked at line T1, resulting in no visible line formation. T2 is a competitive inhibition line where anchored cryptococcal antigen binds unbound antibody, which also fails to form in prozone situations, as all free antibody is saturated with excessive cryptococcal antigen. Lastly, the C (control) line uses anchored antibody to bind control antibody, which does not interact with cryptococcal antigen and allows line formation in cases of extreme antigen burden. The result is a CrAgSQ assay with no line at T1 or T2, but a visible line at C only, resulting in a 5+ grade (Fig. 1). Of note, this is different than a negative result where a line forms at T2 and C. Thus, the CrAgSQ is specifically designed to detect the prozone effect and successfully did so in our study.
Increasing CrAgSQ semiquantitative grades predicted greater CrAg LFA titer and quantitative cryptococcal culture results. With data suggesting CSF CrAg LFA titers ≥1:1,280 are associated with increased 2-week and 10-week mortality (9), the CrAgSQ provides important point-of-care prognostic information, where grades ≥3+ suggest a patient may be at higher risk for poor outcome. Indeed, our study demonstrated increased proportions of participants who died within 14 days as CrAgSQ grade increased (5% [1/22] in grades 1 to 3+ versus 21% [8/38] in grades 4 to 5+). Performing CrAg titers, although simple, is labor intensive, and each dilution increases cost. The highest CrAg titer in this study required 24 dilutions, and the average CrAg-positive sample required 13 dilutions, including a final negative dilution. The CrAgSQ provides a semiquantitative result in a single step test, which provides immediate risk stratification for patients with cryptococcal meningitis.
While the diagnostic performance of the CrAgSQ was excellent, one limitation is its interpretation system is complicated, requiring trained and proficient laboratory staff. While CrAg LFA is a simple test that can be performed at the bedside and requires minimal training, the CrAg is a moderately complex test, which may be challenging for nontrained staff to interpret. Our study used a single-blinded reader to evaluate CrAgSQ grades, and while this individual is highly experienced with this assay, previous studies have shown interreader variability complicates this assay (11). We had a relatively short follow-up period of 14 days for survival outcome and thus cannot comment on how CrAgSQ might predict longer-term survival in cryptococcal meningitis patients. Additionally, the time-to-event survival analysis was underpowered to detect a statistically significant difference. We did not have prespecified power calculations for either diagnostic accuracy or survival, as our study cohort was dictated by availability of novel test supplies over a limited enrollment period.
The CrAgSQ is an improved diagnostic test for cryptococcosis compared to the current FDA-approved CrAg LFA (IMMY). By giving a semiquantitative test result in a single step test, the CrAgSQ is more economical, saving the use of additional test strips. The CrAgSQ may also prevent false-negative results by overcoming the prozone phenomenon, yet the CrAgSQ LFA maintained sensitivity in low-antigen concentrations, which other semiquantitative CrAg assays have not maintained (11). Overall, the CrAgSQ should be implemented for the diagnosis of cryptococcosis from the CSF and provides the benefit of additional prognostic clinical information at the point of care.
ACKNOWLEDGMENTS
We are grateful for institutional support from the Infectious Diseases Institute and the patients of the Mulago Hospital system who consented to study participation.
We declare no conflict of interest. The manufacturers of the diagnostic tests had no role in study design, data analysis, or writing of the manuscript.
This research was made possible through support from the National Institute of Allergy and Infectious Diseases (T32AI055433, U01AI089244, and K23AI138851), National Institute of Neurologic Disorders and Stroke (R01NS086312), the Fogarty International Center (K01TW010268), and a combined National Institute of Neurologic Disorders and Stroke and Fogarty International Center award (D43TW009345) via the Northern Pacific Global Health Fellows Program. R.K. and D.B.M. are currently supported through the DELTAS Africa Initiative grant number DEL-15-011 to THRiVE-2, from Wellcome Trust grant number 107742/Z/15/Z, and the UK government.
Footnotes
Supplemental material is available online only.
Contributor Information
David R. Boulware, Email: skipp015@umn.edu.
Caleb P. Skipper, Email: skipp015@umn.edu.
Kimberly E. Hanson, University of Utah
REFERENCES
- 1.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR. 2017. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17:873–881. 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkes-Ratanshi R, Achan B, Kwizera R, Kambugu A, Meya D, Denning DW. 2015. Cryptococcal disease and the burden of other fungal diseases in Uganda; where are the knowledge gaps and how can we fill them? Mycoses 58(Suppl 5):85–93. 10.1111/myc.12387. [DOI] [PubMed] [Google Scholar]
- 3.Kwizera R, Bongomin F, Lukande R. 2020. Deep fungal infections diagnosed by histology in Uganda: a 70-year retrospective study. Med Mycol 58:1044–1052. 10.1093/mmy/myaa018:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulware DR, Rolfes MA, Rajasingham R, von Hohenberg M, Qin Z, Taseera K, Schutz C, Kwizera R, Butler EK, Meintjes G, Muzoora C, Bischof JC, Meya DB. 2014. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis 20:45–53. 10.3201/eid2001.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letang E, Muller MC, Ntamatungiro AJ, Kimera N, Faini D, Furrer H, Battegay M, Tanner M, Hatz C, Boulware DR, Glass TR. 2015. Cryptococcal antigenemia in immunocompromised human immunodeficiency virus patients in rural Tanzania: a preventable cause of early mortality. Open Forum Infect Dis 2:ofv046. 10.1093/ofid/ofv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyene T, Zewde AG, Balcha A, Hirpo B, Yitbarik T, Gebissa T, Rajasingham R, Boulware DR. 2017. Inadequacy of high-dose fluconazole monotherapy among cerebrospinal fluid cryptococcal antigen (CrAg)-positive human immunodeficiency virus-infected persons in an Ethiopian CrAg screening program. Clin Infect Dis 65:2126–2129. 10.1093/cid/cix613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajasingham R, Wake RM, Beyene T, Katende A, Letang E, Boulware DR. 2018. Cryptococcal meningitis diagnostics and screening in the era of point-of-care laboratory testing. J Clin Microbiol 57:e01238-18. 10.1128/JCM.01238-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wake RM, Britz E, Sriruttan C, Rukasha I, Omar T, Spencer DC, Nel JS, Mashamaite S, Adelekan A, Chiller TM, Jarvis JN, Harrison TS, Govender NP. 2018. High cryptococcal antigen titers in blood are predictive of subclinical cryptococcal meningitis among human immunodeficiency virus-infected patients. Clin Infect Dis 66:686–692. 10.1093/cid/cix872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kabanda T, Siedner MJ, Klausner JD, Muzoora C, Boulware DR. 2014. Point-of-care diagnosis and prognostication of cryptococcal meningitis with the cryptococcal antigen lateral flow assay on cerebrospinal fluid. Clin Infect Dis 58:113–116. 10.1093/cid/cit641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mpoza E, Mukaremera L, Kundura DA, Akampurira A, Luggya T, Tadeo KK, Pastick KA, Bridge SC, Tugume L, Kiggundu R, Musubire AK, Williams DA, Muzoora C, Nalintya E, Rajasingham R, Rhein J, Boulware DR, Meya DB, Abassi M. 2018. Evaluation of a point-of-care immunoassay test kit 'StrongStep' for cryptococcal antigen detection. PLoS One 13:e0190652. 10.1371/journal.pone.0190652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skipper C, Tadeo K, Martyn E, Nalintya E, Rajasingham R, Meya DB, Kafufu B, Rhein J, Boulware DR. 2020. Evaluation of serum cryptococcal antigen testing using two novel semiquantitative lateral flow assays in persons with cryptococcal antigenemia. J Clin Microbiol 58:e02046-19. 10.1128/JCM.02046-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwizera R, Omali D, Tadeo K, Kasibante J, Rutakingirwa MK, Kagimu E, Ssebambulidde K, Williams DA, Rhein J, Boulware D, Meya DB. 2020. Evaluation of the Dynamiker cryptococcal antigen lateral flow assay for the diagnosis of HIV-associated cryptococcosis. J Clin Microbiol 59:e02421-20. 10.1128/JCM.02421-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyal J, Akampurira A, Rhein J, Morawski BM, Kiggundu R, Nabeta HW, Musubire AK, Bahr NC, Williams DA, Bicanic T, Larsen RA, Meya DB, Boulware DR, on behalf of the ASTRO-CM Trial Team. 2016. Reproducibility of CSF quantitative culture methods for estimating rate of clearance in cryptococcal meningitis. Med Myco 54:361–369. 10.1093/mmy/myv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvis JN, Tenforde MW, Lechiile K, Milton T, Boose A, Leeme TB, Tawe L, Muthoga C, Rukasha I, Mulenga F, Rulaganyang I, Molefi M, Molloy SF, Ngidi J, Harrison TS, Govender NP, Mine M. 2020. Evaluation of a novel semiquantitative cryptococcal antigen lateral flow assay in patients with advanced HIV disease. J Clin Microbiol 58:e00441-20. 10.1128/JCM.00441-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lourens A, Jarvis JN, Meintjes G, Samuel CM. 2014. Rapid diagnosis of cryptococcal meningitis by use of lateral flow assay on cerebrospinal fluid samples: influence of the high-dose “hook” effect. J Clin Microbiol 52:4172–4175. 10.1128/JCM.01683-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download JCM.00860-21-s0001.pdf, PDF file, 100 KB (99.3KB, pdf)