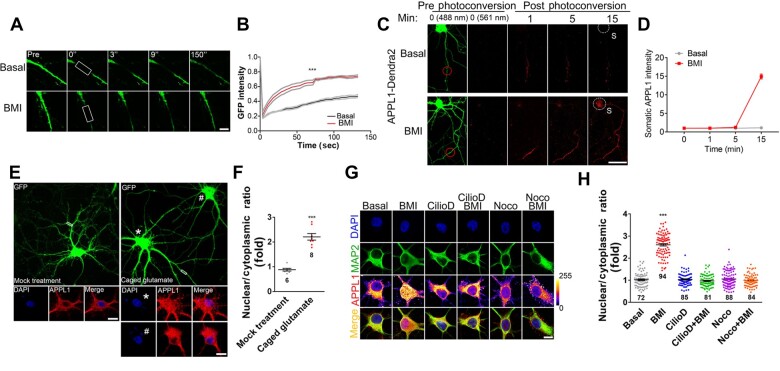
Figure 2.
APPL1 undergoes neuronal activity-induced retrograde transport along the dendrites. (A and B) APPL1-GFP was transfected into hippocampal neurons at DIV 12 for 24 h before FRAP experiment. Neurons were incubated in Tyrode’s solution containing Torlox (10 nM) at 37°C and subsequently stimulated with BMI/4-AP (50 μM/2.5 mM) for 5 min before photobleaching. Distal dendrites of GFP-positive neurons were selected as ROIs (white rectangle) for analysis. FRAP curves for APPL1-GFP fluorescence in total 150 sec are shown. The average fluorescence before photobleaching was counted as 1.0. Gray lines indicate the mean fluorescence intensity ±SEM. Scale bar, 10 μm. (C) Dendra2-tagged APPL1 was transfected into hippocampal neurons at DIV 12 for 24 h before photoswitching experiment. Neurons were incubated at 37°C in Tyrode’s solution containing Torlox (10 nM) and subsequently treated with BMI (50 μM). Photoswitch of Dendra2 was performed by UV (405 nm) at the distal dendrites (ROI; red circle) of Dendra2-positive (488 nm) neurons. Images were taken in the red (561 nm) fluorescent channel, and signals were acquired every 1 min for 15 min. Red fluorescence in white-dotted circle indicates nuclear accumulation of APPL1. Scale bar, 100 μm. (D) Statistical analysis of somatic APPL1-Dendra2. (E) Hippocampal neurons were infected with lentivirus expressing EGFP at DIV 6, and 7 days later, MNI-caged glutamate (200 μM, right panel) or vehicle (as control, left panel) was applied and then uncaged at ROIs (white rectangle) of GFP-positive neurons under UV pulse. After incubation for 30 min, neurons were fixed and stained with antibodies against APPL1 (red) and with DAPI nuclear dye (blue). Scale bar, 10 μm. (F) Statistical analysis of nuclear/cytoplasmic ratio of APPL1 with or without glutamate stimulation (* indicates the neuron with caged glutamate; # indicates the neighbor neuron without caged glutamate). (G and H) Cultured hippocampal neurons were untreated (basal) or treated with Ciliobrevin D (Cilio D, 0.5 mM) or Nocodazole (Noco, 10 μg/ml), respectively, for 1 h. Alternatively, hippocampal neurons were pretreated with Ciliobrevin D or Nocodazole, respectively, for 30 min followed by treatment with BMI for another 1 h. After treatment, the cultures were immunostained with antibodies against MAP2 (green) and APPL1 (color lookup table, pixel intensities from 0 to 255) and with DAPI nuclear dye (blue). Scale bar, 10 μm. Data are presented as mean ± SEM. ***P < 0.005.