Purpose of review
The purpose of this review is to discuss the clinical management of children with pediatric rheumatic disease (PRD) during the Coronavirus disease of 2019 (COVID-19) pandemic, as well as the unique role of the pediatric rheumatologist during a time of emerging post-COVID inflammatory sequelae including, multisystem inflammatory syndrome in children (MIS-C).
Recent findings
To date, there has been little evidence to suggest that children with PRD, including those on immunomodulatory therapies, are at increased risk for severe COVID-19. Clinical guidance statements have been created to support clinical providers in providing care to children with PRD during the COVID-19 pandemic. Pediatric rheumatologists have also been called upon to assist in the identification and management of post-COVID sequelae, including the rapidly emerging inflammatory illness, MIS-C.
Summary
The COVID-19 era has been defined by a rapid expansion in scientific knowledge and a time of extraordinary local and worldwide collaboration, both within the pediatric rheumatology community, as well as across multiple disciplines. Through collective efforts, we have learned that children with PRD, including those on immunomodulatory therapies, are not at increased risk for severe COVID-19. Pediatric rheumatologists have also worked alongside other disciplines to develop guidance for the management of MIS-C, with the majority of patients experiencing excellent clinical outcomes.
Keywords: COVID-19, multisystem inflammatory syndrome in children (MIS-C), pediatric rheumatic disease
INTRODUCTION
The global spread of Coronavirus disease of 2019 (COVID-19) abruptly impacted the pediatric rheumatology community, prompting physicians to rapidly assess the impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children with pediatric rheumatic disease (PRD) and the implications of immunosuppressive treatment on their risk for severe disease. Numerous questions arose surrounding preventive measures, risk reduction, ongoing immunosuppressive management, and methods to minimize disruption to clinical care. Simultaneously, pediatric rheumatologists quickly found themselves amidst the discovery of an unanticipated inflammatory syndrome related to COVID-19, now termed multisystem inflammatory syndrome in children (MIS-C). In a multidisciplinary collaborative effort with pediatric physicians including infectious disease and cardiology, pediatric rheumatologists have assisted in providing guidance surrounding the diagnostic evaluation and medical management of MIS-C. In this review, we will discuss the impact and clinical management of children with PRD during the COVID-19 pandemic, review the literature related to the clinical presentation and management of MIS-C, and briefly discuss unique clinical sequelae of COVID-19 that may prompt evaluation by a pediatric rheumatologist.
Box 1.
no caption available
THE IMPACT OF COVID-19 IN CHILDREN WITH PEDIATRIC RHEUMATIC DISEASE
Since the onset of the COVID-19 pandemic, there have been concerns raised related to the potential impact of SARS-CoV-2 infection in patients with rheumatic disease and patients on immunosuppressive therapy. Reports in the literature of patients with adult-onset rheumatic disease have demonstrated that advancing age and underlying comorbidities remain a primary factor in the risk for severe complications from COVID-19, similar to the general population [1▪▪,2▪▪]. Few studies have also described disease-specific factors including underlying rheumatic disease, disease activity, the presence of lung involvement and certain immunomodulatory medications (including corticosteroids, rituximab and conventional disease modifying antirheumatic drugs (DMARDs)) that may additionally predict worse outcomes [2▪▪,3,4▪]. However, to date, there is little evidence to suggest a higher risk for severe COVID-19 in children with PRD [5–9] or children receiving immunomodulatory therapies commonly used for PRD [10–15].
In May 2020, the American College of Rheumatology (ACR) developed the ACR COVID-19 Clinical Guidance for Pediatric Rheumatology Task Force, charged to provide clinical guidance to rheumatology providers who treat children with PRD in the context of the COVID-19 pandemic [16▪]. Recognizing that children with PRD do not appear to be at significantly increased risk of severe COVID-19 and acknowledging the need to take into account individual patient characteristics and prevalence of SARS-CoV-2 transmission in the community, these general recommendations were aimed at assuring optimal control of underlying PRD during the era of the COVID-19 pandemic. Guidance was provided to discourage physicians from modifying or delaying immunomodulatory therapy in the absence of SARS-CoV-2 exposure or infection. Similarly, in the presence of close/household exposure or asymptomatic COVID-19, recommendations were to continue medical therapy needed to control underlying PRD, with special consideration to reduce corticosteroid burden to the lowest effective dose possible to control underlying disease. Concerns related to the use of rituximab and cyclophosphamide raised by the Global Rheumatology Alliance in adults with rheumatic disease [1▪▪] were acknowledged; however, given the overall reduced risk of severe COVID-19 in the pediatric population and the fact that these medications are typically reserved for severe life and/or organ threatening disease in children, the task force agreed that the benefits of continuing therapy likely outweigh the risk in most cases [16▪]. In contrast, in the presence of symptomatic COVID-19, the task force agreed to conform to conventional practices related to concurrent infections and recommended holding all DMARDs for the duration of symptoms and up to 7–14 days after resolution of fever and respiratory symptoms. Special considerations were made for patients with PRD on interleukin (IL)-1 inhibitors, as these patients may be particularly sensitive to medication disruptions and with the knowledge that selective IL-1 inhibitors have been safely used in other infections [17].
Another consideration that the pediatric rheumatologist faced in the era of the COVID-19 pandemic is the responsibility to assure adequate and timely access to clinical care, particularly during times of increased community transmission of SARS-CoV-2. In evaluating clinical practice and patient perspectives during the COVID-19 pandemic, both patients and families acknowledged that apprehension about in-person clinical assessments and safe access to the hospital system [18▪] may have resulted in delays to care and exacerbation of underlying illness [19]. As a result, the rapid expansion of telemedicine during this time has been instrumental in improving access to care for children with PRD [19–22], with the development of comprehensive telemedicine assessments, including standardizing the musculoskeletal physical exam using the video version of paediatric Gait Arms Legs and Spine (pGALS), V-pGALS [23▪]. Despite the numerous benefits of telemedicine, several limitations should also be acknowledged, specifically related to the quality and comprehensiveness of care, psychosocial evaluation, and the availability of access to technology to maintain health equity [21,24].
In addition to concerns related to medical management of children with PRD, the COVID-19 pandemic has also raised awareness of the impact emotional distress, school closures, and limited socialization on the overall well being of children with chronic illness. Children and adolescents with PRD have a relatively high prevalence of anxiety and depression at baseline compared to the general pediatric population [25,26]. Furthermore, there is evidence that patients within the Black and Latinx populations may be disproportionately impacted by the pandemic from both a medical and psychosocial standpoint [18▪,27–30]. Pediatric rheumatology providers must be mindful of the burden of the COVID-19 pandemic on both children with PRD and their caregivers, recognizing the impact of psychosocial distress on physical disease, and assist with referrals to mental health services. Similarly, with regards to in-person schooling, the ACR COVID-19 Clinical Guidance for Pediatric Rheumatology Task Force recognized the generally low rates of transmission in primary and secondary schools [31] and emphasized the benefits of attending in-person school, once taking into account individual patient characteristics and comorbidities. Finally, to date, there has been no evidence to suggest children with PRD are at higher risk of adverse reactions from the COVID-19 vaccine and thus the task force recommended that children, adolescents, and young adults with PRD should receive the vaccine in accordance with Centers for Disease Control and local recommendations.
MULTI-INFLAMMATORY SYNDROME IN CHILDREN: THE ROLE OF THE PEDIATRIC RHEUMATOLOGIST
Since first described in Europe in April 2020, MIS-C (also known as pediatric multisystem inflammatory syndrome (PMIS)), has been increasingly recognized throughout the world. The presentation of MIS-C is temporally linked to COVID-19 exposure, with peaks of disease typically following surges of COVID-19 cases by approximately 3–6 weeks, and patients demonstrating evidence of prior SARS-CoV-2 infection with positive IgG serologies. Although MIS-C remains an overall rare condition, clinical presentations with cardiogenic shock and multiorgan dysfunction have created an impetus for rapid multidisciplinary collaboration and strategies to guide clinical management. Given the presentation of MIS-C as a systemic inflammatory condition with clinical symptomatology that often overlaps with Kawasaki disease, pediatric rheumatologists have been involved from the onset in attempts to understand the underlying pathophysiology, and have assisted in developing guidance for diagnostic evaluation, clinical monitoring and management with immunomodulatory therapy [32▪▪,33].
In the year since the initial description of MIS-C, knowledge regarding the presentation, management, and pathophysiology has rapidly expanded; however, numerous uncertainties remain. The majority of children presenting with MIS-C are previously healthy, with reports of prior asymptomatic or minimally symptomatic COVID-19; thus, underlying predisposing factors for MIS-C remain unknown. There is a range of clinical severity, yet a majority of patients require care in pediatric intensive care units (ICUs) during their hospitalization. Furthermore, while MIS-C was initially described as ‘Kawasaki-like’, numerous reports have studied these overlapping phenotypes and have since described differences in both clinical and immunochemical presentations between MIS-C and Kawasaki disease [34▪▪,35▪,36▪].
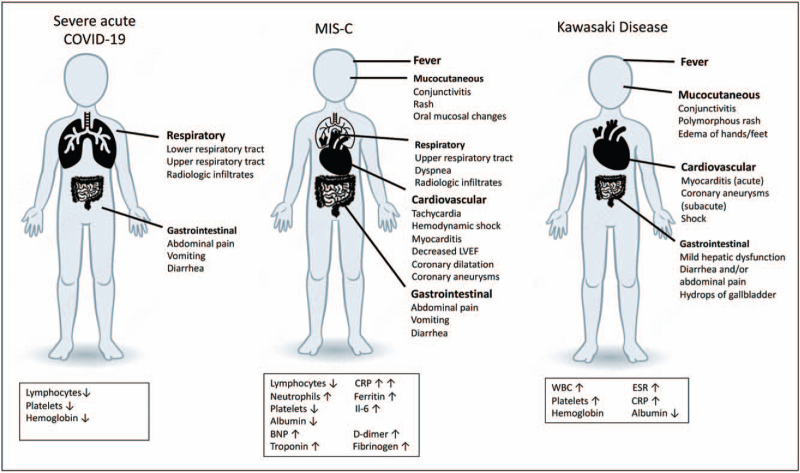
Current literature on MIS-C is represented by case reports, case series, and systematic reviews. In one of the largest systematic review to date, Hoste et al. summarized articles published on MIS-C/PMIS cases from December 2019 through August 2020 [34▪▪]. Median age at presentation was 8.4 years, with a large proportion of patients of Black (37%) and Latino/Hispanic (29%) descent. With the exception of obesity (25%), other comorbidities were rare. Nearly, all patients presented with fever (99%) and most commonly involved organ systems included gastrointestinal (86%), cardiovascular (79%), and respiratory (50%) (Fig. 1). In this systematic review, 23% of patients fulfilled criteria for complete Kawasaki disease, whereas 24.1% resembled incomplete Kawasaki disease. Laboratory evidence of systemic inflammation was evident in the majority of MIS-C cases, with elevated acute phase reactants including C-reactive protein, IL-6, and ferritin. Patients also had significantly elevated markers of coagulation (D-dimer, fibrinogen) and myocardial injury (troponin, brain natriuretic peptide).
FIGURE 1.
Summary figure of main clinical and laboratory findings in pediatric acute severe COVID-19 disease, MIS-C and Kawasaki disease. CRP, C reactive protein; ESR, erythrocyte sedimentation rate; IL-6, interleukin-6; LVEF, left ventricular ejection fraction.
Many investigators have compared children with MIS-C to historical Kawasaki disease patients. MIS-C patients tend to have a broader age range, with a median age range higher than that of classic Kawasaki disease (median age: 2–2.7 years) [34▪▪,35▪]. MIS-C patients are less likely to have coronary artery abnormalities and more likely to have ventricular dysfunction [35▪]. Evidence of systemic inflammation also appears to be substantially higher in MIS-C compared to historical Kawasaki disease cohorts. Studies have additionally compared clinical presentation, laboratory markers and outcomes in children and adolescents with MIS-C and acute COVID-19 [37,38]. Feldstein et al. compared cases of MIS-C in a USA cohort with severe acute COVID-19 and found that patients with MIS-C were more likely to be 6–12 years old, non-Hispanic/Black and more likely to be previously healthy [38]. Clinical symptoms that distinguished MIS-C patients from acute COVID-19 included mucocutaneous symptoms and severe cardiovascular involvement without respiratory involvement. Patients with MIS-C were more likely to have higher neutrophil-to-lymphocyte ratios, higher CRP levels, and lower platelet counts [38]. A summary of clinical and laboratory features of MIS-C, Kawasaki disease, and acute COVID-19 is presented in Fig. 1.
To date, immunomodulatory management of MIS-C has focused on its resemblance with Kawasaki disease and theoretical concerns about cardiac sequelae and potential coronary artery aneurysms, similar to those described with untreated KD. While data are limited to retrospective reports, case series, and anecdotal evidence, most reports describe widespread use of intravenous immunoglobulin (IVIG) and corticosteroids in 50–90% of patients for the treatment of MIS-C, with variability noted in treatment strategies and dosing among different centers and studies [33,34▪▪,35▪] One retrospective cohort study from France [39] compared use of IVIG alone versus IVIG plus methylprednisolone as initial therapy for MIS-C in propensity score-matched cohorts and found that combined therapy was associated with improved outcomes, including lower rate of treatment failure, lower use of second-line treatment, less need for hemodynamic support, less evidence of acute left ventricular dysfunction, and shorter ICU stay. IL-1 inhibition with anakinra has also been described in refractory disease in up to 8–26% of patients [34▪▪,35▪]. It is worth noting that a minority of patients in several case series have self-resolving inflammation and did not require immunomodulatory therapy. Other agents used less frequently include IL-6 inhibitors and tumor necrosis factor inhibitors [34▪▪]. Hoste et al. report the use of additional supportive care including inotropic agents (55%), mechanical ventilation (24%), noninvasive ventilation (26%), and extracorporeal membrane oxygenation support (4%) [34▪▪]. Although a majority of patients require ICU support (73%), almost all patients have excellent outcomes, with very few deaths reported and minimal long-term sequelae [40].
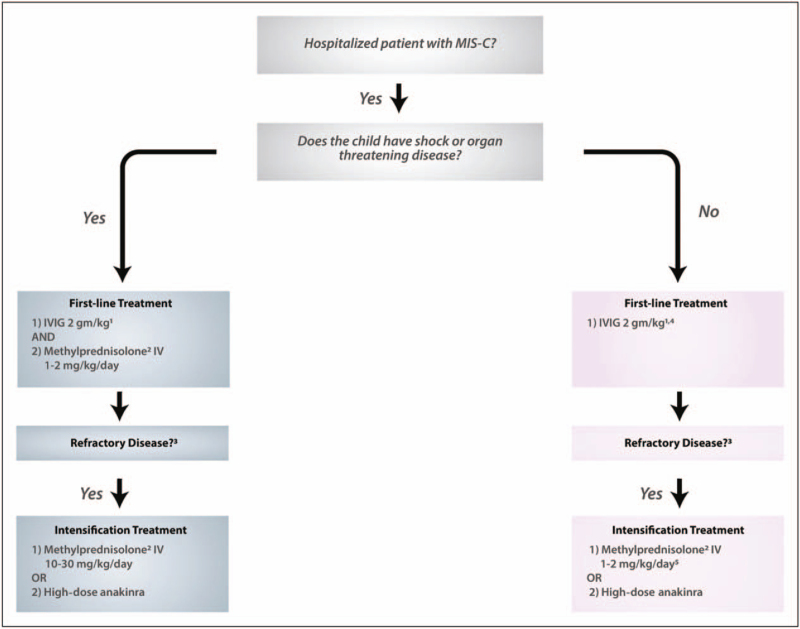
The ACR published clinical guidance for treatment of MIS-C and hyperinflammation in COVID-19 in June 2020 with revisions published in November 2020 [32▪▪]. This document includes a diagnostic pathway for MIS-C and a discussion of features distinguishing MIS-C from KD. Clinical guidance is provided for laboratory evaluation and cardiac monitoring, stratified by clinical presentation. Guidance for immunomodulatory therapy recommends stepwise approach with initiation of IVIG for all children hospitalized with MIS-C and/or fulfil Kawasaki disease criteria with careful consideration of cardiac function and fluid status to prevent fluid overload. Corticosteroids are recommended as first-line therapy for shock or organ threatening disease, or as a second line therapy for refractory disease (Fig. 2). Other treatment options for refractory disease include high dose intravenous corticosteroids and IL-1 inhibition [32▪▪].
FIGURE 2.
American College of Rheumatology (ACR) algorithm initial immunomodulatory treatment in MIS-C (original legend included in image). Algorithm for initial immunomodulatory treatment of multisystem inflammatory syndrome in children (MIS-C). Moderate-to-high consensus was reached by the Task Force in the development of this treatment algorithm for MIS-C associated with severe acute respiratory syndrome coronavirus 2. 1Intravenous immunoglobuin (MG) dosing is 2 gm/kg based on ideal body weight. Cardiac function and fluid status should be assessed before MG is given. In some patients with cardiac dysfunction, MG may be given in divided doses (1 gm/kg da1y over 2 days). 2Methylpremisolone or another steroid at equivalent dosing may be used. 3Refractorry disease is defined as persistent fevers and/or ongoing and significant end-organ involvement. 4Low-to-moderate-dose glucocorticoids (methylprednisolone 1-2 mg/kg/day) may be considered for first-line therapy in some MIS-C patients with concerning features (ill appearance, highly elevated B-type natriuretic peptide levels, unexplained tachycardia) who have not yet developed shock or organ-threatening disease. 5If the patient was given Iow-to-moderate-dose glucocorticoids as first-line therapy, methylprednisolone IV dosing should be 10–30 mg/kg/day for intensification treatment. Reproduced with permission from Henderson et al. [32▪▪].
POST-COVID SEQUELAE
In addition to acute COVID-19 infection and postinfectious inflammatory conditions, post-COVID sequelae have also been described and are also being addressed by pediatric rheumatologists worldwide. Regardless of symptoms at diagnosis or severity of acute infection, several patients suffer from long-term effects of COVID-19. Rheumatologists are being called upon to assess a wide array of symptoms commonly seen as presentations of systemic autoimmune disease and to distinguish them from lingering effects of COVID-19. Symptoms vary among studies with fatigue, muscle weakness, sleep difficulties, and anxiety or depression commonly reported as long-term effects [41]. In a meta-analysis of studies world-wide, fatigue, headache, attention disorder, hair-loss, and dyspnea were the most common symptoms [42]. Although myalgia was the most common musculoskeletal symptom reported during acute COVID infection, many musculoskeletal findings have been reported as post-COVID complications including myositis, neuropathy, arthropathy, and soft tissue abnormalities [43]. In children, data are scarce regarding long-term consequences of COVID-19 with few reports of fatigue, dyspnea, and heart palpitations or chest pain. Other symptoms reported include decreased concentration, prolonged fevers, and headaches [44].
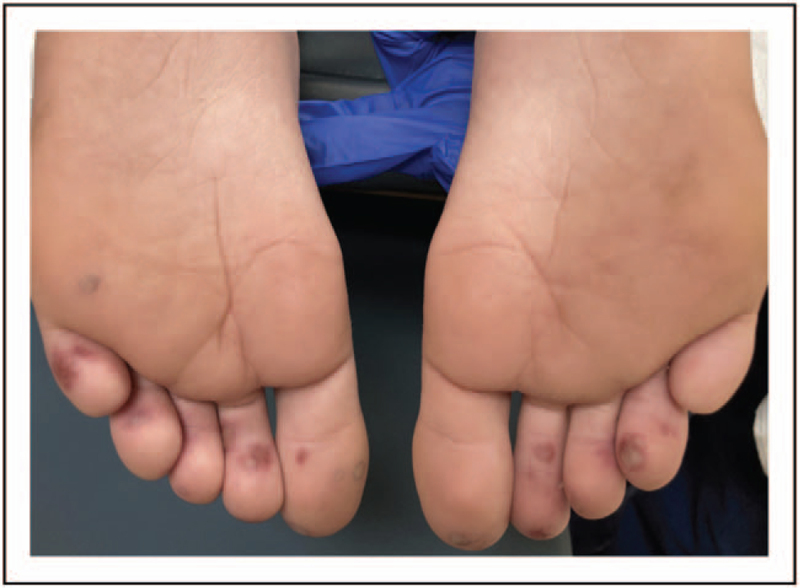
Additional clinical features seen in both acute infection with COVID-19 and post-COVID sequelae are dermatologic manifestations that may mimic common rheumatologic conditions. The most common dermatologic findings in acute COVID-19 infection are maculopapular rash, urticaria, chilblains vesicular lesions, livedo reticularis, and petechiae. Interestingly, an increased incidence of chilblains has been reported during the pandemic and found to be more common in younger age patients [45]. Chilblains seem to appear after the active phase of disease, most commonly described in patients who were asymptomatic carriers of COVID -19 and found to have antibodies only after chilblains was reported. This entity has been termed ‘COVID toes’ and has been described in both adults and children (Fig. 3). Most pediatric cases present with no evidence of acute COVID-19 infection, and often with negative SARS-CoV-2 PCR testing and negative antibodies [46]. Histopathologic studies of these lesions have shown variable degrees of lymphocytic vasculitis with evidence of endothelial damage [47▪]. Coronavirus particles have also been described within the endothelium [47▪]. Prognosis of children with chilblains is favorable, with spontaneous regression within 2–8 weeks as the most common outcome. In severe cases, a trial of topical steroids and/or oral antihistamines may be considered [46,48].
FIGURE 3.
Clinical presentations of chilblains in an otherwise healthy young boy, presenting with pain and swelling in affected toes.
CONCLUSION
Despite the numerous challenges to the medical community, the COVID-19 era has been defined by a rapid expansion in scientific knowledge and a time of extraordinary local and worldwide collaboration, both within the pediatric rheumatology community, as well as across multiple disciplines. Through collective efforts, we have learned that children with PRD, including those on immunomodulatory therapies, are not at increased risk for severe COVID-19; thus, the overall goals in the management of patients with PRD include continued therapy to assure prompt control of active disease, relief of symptoms, and prevention of long-term sequelae. Pediatric rheumatology providers must be mindful of challenges that have been exacerbated during the pandemic including healthcare inequities, impaired access to clinical care and psychosocial distress. Finally, pediatric rheumatology providers have been called upon to assist in the management of post-COVID sequelae, including MIS-C, relying on expertise in multidisciplinary collaboration, knowledge of immune responses, and the use of immunomodulatory therapies.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪▪.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020; 79:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the first reports from the international Global Rheumatology Alliance, discussing the outcomes of the first 600 cases of patients with rheumatic disease enrolled in the registry from 40 countries.
- 2▪▪.Strangfeld A, Schafer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2021; 80:930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent report from the Global Rheumatology Alliance providing further insight on risk factors of severe disease in adult patients with rheumatic disease.
- 3.Bower H, Frisell T, Di Giuseppe D, et al. Impact of the COVID-19 pandemic on morbidity and mortality in patients with inflammatory joint diseases and in the general population: a nationwide Swedish cohort study. Ann Rheum Dis 2021; doi: 10.1136/annrheumdis-2021-219845. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪.Schafer M, Strangfeld A, Hyrich KL, et al. Response to: ’Correspondence on ’Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician reported registry’ by Mulhearn et al. Ann Rheum Dis 2021; doi: 10.1136/annrheumdis-2021-220134. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; A subanalysis of data from the Global Rheumatology Alliance ellaborating on the interaction between corticosteroids and disease activity on the risk of COVID-19 related mortality in patients with rheumatic disease.
- 5.Yildiz M, Haslak F, Adrovic A, et al. The frequency and clinical course of COVID-19 infection in children with juvenile idiopathic arthritis. Clin Exp Rheumatol 2020; 38:1271–1272. [PubMed] [Google Scholar]
- 6.Kasap Cuceoglu M, Batu ED, Bilginer Y, Ozen S. COVID-19 in paediatric rheumatology patients treated with b/tsDMARDs: a cross-sectional patient survey study. Ann Rheum Dis 2020; doi: 10.1136/annrheumdis-2020-218341. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Gheita TA, Salem MN, Eesa NN, et al. Rheumatologists’ practice during the Coronavirus disease 2019 (COVID-19) pandemic: a survey in Egypt. Rheumatol Int 2020; 40:1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haslak F, Yildiz M, Adrovic A, et al. Management of childhood-onset autoinflammatory diseases during the COVID-19 pandemic. Rheumatol Int 2020; 40:1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvo C, Udaondo C. the Rheumatic Diseases E-AEPWG. COVID-19 in children with rheumatic diseases in the Spanish National Cohort EPICO-AEP. J Rheumatol 2021; doi: 10.3899/jrheum.201548. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10.Marino A, Romano M, Gattinara M, Cimaz R. Patients with juvenile idiopathic arthritis on TNF inhibitors exposed to COVID-19 family members. Semin Arthritis Rheum 2020; 50:1214–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Arcangelo G, Distante M, Raso T, et al. Safety of biological therapy in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2021; 72:736–741. [DOI] [PubMed] [Google Scholar]
- 12.Bossa F, Carparelli S, Latiano A, et al. Impact of the COVID-19 outbreak and the serum prevalence of SARS-CoV-2 antibodies in patients with inflammatory bowel disease treated with biologic drugs. Dig Liver Dis 2021; 53:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marlais M, Wlodkowski T, Al-Akash S, et al. COVID-19 in children treated with immunosuppressive medication for kidney diseases. Arch Dis Child 2020; doi: 10.1136/archdischild-2020-320616. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner D, Huang Y, Martin-de-Carpi J, et al. Corona Virus Disease 2019 and Paediatric Inflammatory Bowel Diseases: Global Experience and Provisional Guidance (March 2020) from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2020; 70:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner EJ, Pigneur B, Focht G, et al. Benign evolution of SARS-Cov2 infections in children with inflammatory bowel disease: results from two international databases. Clin Gastroenterol Hepatol 2021; 19: 394.e5-396.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪.Wahezi DM, Lo MS, Rubinstein TB, et al. American College of Rheumatology Guidance for the Management of Pediatric Rheumatic Disease During the COVID-19 Pandemic: Version 1. Arthritis Rheumatol 2020; 72:1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]; A multidisciplinary collaboration charged to provide clinical guidance on the care of children with pediatric rheumatic disease during the COVID-19 pandemic.
- 17.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 2016; 44:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪.Maldonado D, Tu E, Mahmood S, et al. Medication access difficulty and COVID-related distress are associated with disease flares in rheumatology patients during the COVID-19 pandemic. Arthritis Care Res (Hoboken) 2020; doi: 10.1002/acr.24531. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; A patient survey conducted during the height of the COVID-19 pandemic demonstrating the impact of the pandemic on medication access and mental health in patients with rheumatic disease.
- 19.Batu ED, Lamot L, Sag E, et al. How the COVID-19 pandemic has influenced pediatric rheumatology practice: results of a global, cross-sectional, online survey. Semin Arthritis Rheum 2020; 50:1262–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bos WH, van Tubergen A, Vonkeman HE. Telemedicine for patients with rheumatic and musculoskeletal diseases during the COVID-19 pandemic; a positive experience in the Netherlands. Rheumatol Int 2021; 41:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akintayo RO, Akpabio AA, Kalla AA, et al. The impact of COVID-19 on rheumatology practice across Africa. Rheumatology (Oxford) 2021; 60:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naveen R, Sundaram TG, Agarwal V, Gupta L. Teleconsultation experience with the idiopathic inflammatory myopathies: a prospective observational cohort study during the COVID-19 pandemic. Rheumatol Int 2021; 41:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪.Shenoi S, Hayward K, Curran ML, et al. Telemedicine in pediatric rheumatology: this is the time for the community to embrace a new way of clinical practice. Pediatr Rheumatol Online J 2020; 18:85. [DOI] [PMC free article] [PubMed] [Google Scholar]; Adaptation of the pediatric musculoskeletal exam (pGALS) during the rapid expansion of telehealth.
- 24.Balmuri N, Onel KB. Glitches in the utilization of telehealth in pediatric rheumatology patients during the COVID-19 pandemic. Pediatr Rheumatol Online J 2020; 18:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fair DC, Rodriguez M, Knight AM, Rubinstein TB. Depression and anxiety in patients with juvenile idiopathic arthritis: current insights and impact on quality of life, a systematic review. Open Access Rheumatol 2019; 11:237–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quilter MC, Hiraki LT, Korczak DJ. Depressive and anxiety symptom prevalence in childhood-onset systemic lupus erythematosus: a systematic review. Lupus 2019; 28:878–887. [DOI] [PubMed] [Google Scholar]
- 27.Gianfrancesco MA, Leykina LA, Izadi Z, et al. Association of race and ethnicity with covid-19 outcomes in rheumatic disease: data from the COVID-19 global rheumatology alliance physician registry. Arthritis Rheumatol 2021; 73:374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czeisler ME, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30. MMWR Morb Mortal Wkly Rep 2020; 69:1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seyahi E, Poyraz BC, Sut N, et al. The psychological state and changes in the routine of the patients with rheumatic diseases during the coronavirus disease (COVID-19) outbreak in Turkey: a web-based cross-sectional survey. Rheumatol Int 2020; 40:1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maguire S, O'Shea F. Social isolation due to the COVID-19 pandemic has led to worse outcomes in females with inflammatory arthritis. Ir J Med Sci 2021; 190:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Xu W, Dozier M, et al. Uncover: the role of children in the transmission of SARS-CoV2: updated rapid review. J Glob Health 2020; 10:021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪▪.Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 2. Arthritis Rheumatol 2021; 73:e13–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]; A multidisciplinary collaboration with pediatric providers in rheumatology, cardiology and infectious disease to provide guidance on the detection and management of patients with MIS-C.
- 33.Elias MD, McCrindle BW, Larios G, et al. Management of multisystem inflammatory syndrome in children associated with COVID-19: a survey from the International Kawasaki Disease Registry. CJC Open 2020; 2:632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪▪.Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr 2021; 180:2019–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the largest systemic reviews to date, providing an overview of the clinical presentation and management in patients with MIS-C.
- 35▪.Lee PY, Day-Lewis M, Henderson LA, et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest 2020; 130:5942–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comparison of clinical and laboratory manifestations between MIS-C and Kawasaki Disease.
- 36▪.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]; A detailed overview of the initial MIS-C cases in the USA.
- 37.Diorio C, Henrickson SE, Vella LA, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest 2020; 130:5967–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021; 325:1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouldali N, Toubiana J, Antona D, et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA 2021; 325:855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penner J, Abdel-Mannan O, Grant K, et al. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: a retrospective cohort study. Lancet Child Adolesc Health 2021; 5:473–482. [DOI] [PubMed] [Google Scholar]
- 41.Huang Y, Pinto MD, Borelli JL, et al. COVID symptoms, symptom clusters, and predictors for becoming a long-hauler: looking for clarity in the haze of the pandemic. medRxiv 2021; doi: 10.1101/2021.03.03.21252086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv 2021; doi: 10.1101/2021.01.27.21250617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramani SL, Samet J, Franz CK, et al. Musculoskeletal involvement of COVID-19: review of imaging. Skeletal Radiol 2021; 1–11. doi: 10.1007/s00256-021-03734-7. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ludvigsson JF. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr 2021; 110:914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int 2020; 2020:1236520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koschitzky M, Oyola RR, Lee-Wong M, et al. Pediatric COVID toes and fingers. Clin Dermatol 2021; 39:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪.Colmenero I, Santonja C, Alonso-Riano M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol 2020; 183:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]; An overview of the link between SARS-CoV-2 infection and chilblains, with description of pathologic features.
- 48.Andina D, Belloni-Fortina A, Bodemer C, et al. Skin manifestations of COVID-19 in children: part 3. Clin Exp Dermatol 2021; 46:462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]