FIGURE 3.
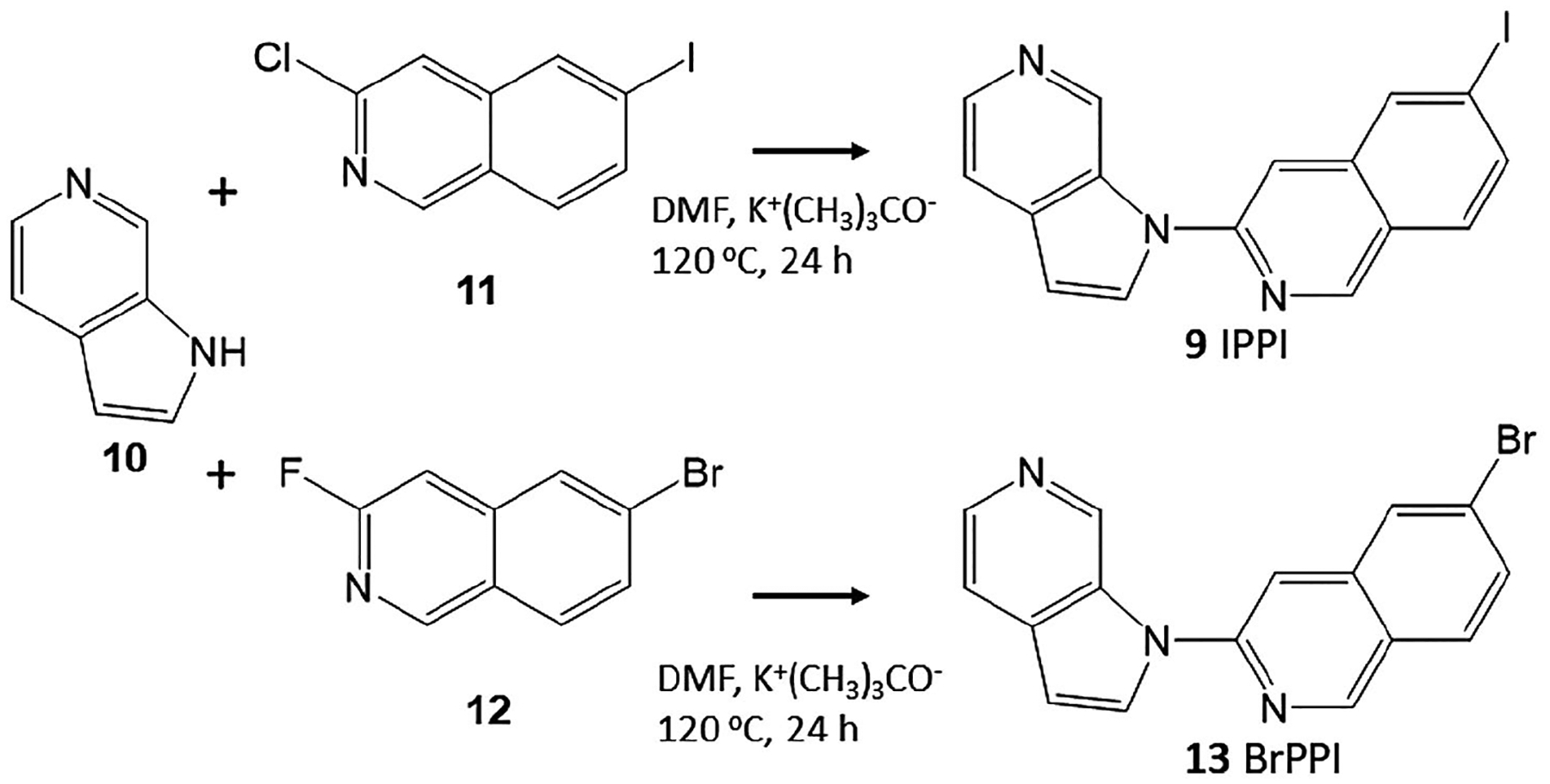
Synthesis of IPPI and BrPPI. Azaindole 10 was reacted with 3-chloro-6-iodoisoquinoline 11 in dimethylformamide (DMF) in the presence of potassium tert-butoxide for 24 hr at 120°C to provide IPPI, 9. Similarly, azaindole 10 was reacted with 3-fluoro-6-bromoisoquinoline 12 in DMF in the presence of potassium tert-butoxide for 24 hr at 120°C to provide 6-bromo-3-(1H-pyrrolo[2,3-c] pyridine-1-yl)isoquinoline, BrPPI, 13
