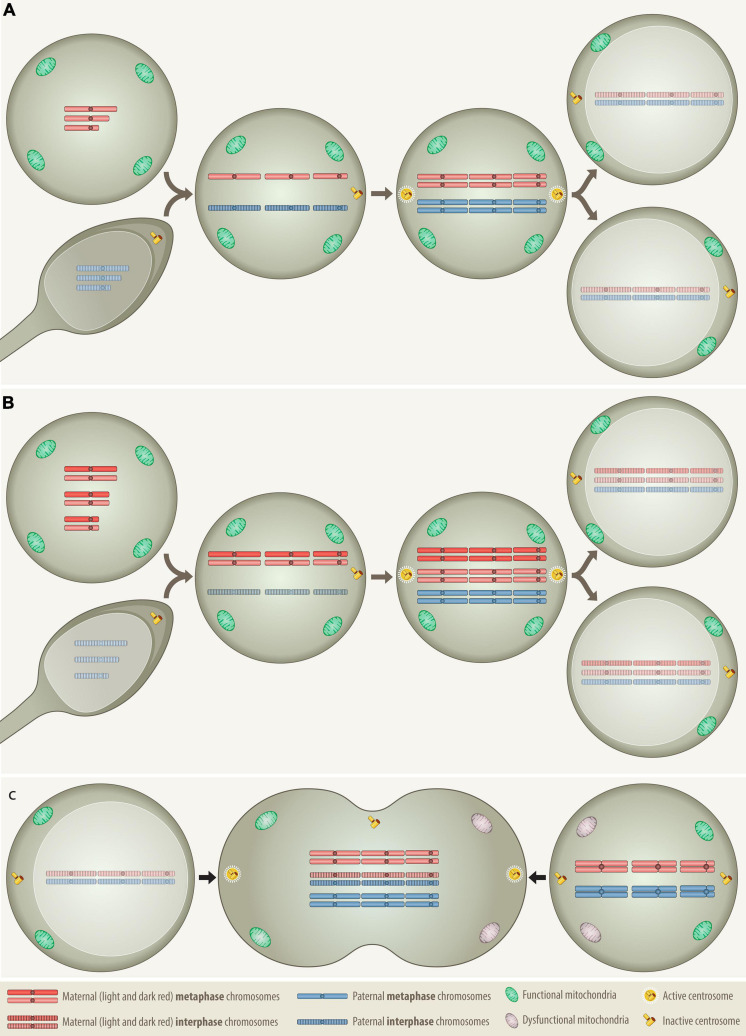
FIGURE 1.
Comparison of the distribution patterns of chromosomes, centrosomes and mitochondria in three different types of cell fusion: (A) a haploid oocyte with a haploid sperm, (B) a diploid oocyte with a haploid sperm, and (C) a mitotic cell with a G0/G1 cell. Fertilization and somatic cell fusion are closely related processes that share many physical and functional properties. To enable the amalgamation of the nuclear material of the involved cells, at least one of the fusion partners needs to be in mitosis, because only a mitotic cell can supply the essential cytoplasmic components that promote the breakdown of nuclear membrane and condense the interphase nucleus into chromatid chromosomes. The combined set of chromosomes must then be accurately allocated to two daughter cells, which is best achieved with the help of a single pair of properly functioning centrosomes. Only daughter cells that receive at least the equivalent of a complete haploid set of chromosomes together with an adequate number of functional mitochondria can survive. (A) In case of normal fertilization, the haploid oocyte provides the power-supplying mitochondria as well as the essential hospital mitotic environment with all the essential components that are necessary to condensate the interphase nucleus of the intruding haploid sperm into individual chromatids, whereas the sperm contributes the prepared centrosome Anlage, which upon fusion is converted into properly functioning centrosomes (Clift and Schuh, 2013). Following duplication of both haploid sets of chromosomes the cell divides and generates the first pair of diploid daughter cells. (B) The formation of a digynic triploidy, which results from the fusion of a diploid oocyte with a haploid sperm, follows essentially the same track. Since digynic triploid zygotes also contain only one single pair of active centrosomes, they can engage in relatively normal mitosis so that all progenitor cells will inherit a stable triploid set of chromosomes. Owing to the consequences of meiotic recombination, homologous chromosomes in diploid oocytes may still contain distinguishable heterozygous regions (Hassold and Hunt, 2001). (C) The somatic type of cell fusion alluded to herein, fuses a mitotic with a G0/G1 interphase cell and resembles therefore in many ways the germline one that produces a digynic triploidy. As the meiotic environment condenses the sperm, the mitotic one will also condense the G0/G1 interphase nucleus into single-stranded chromatid chromosomes, a phenomenon that in the somatic setting is known as “premature chromosome condensation (PCC)” (Ravi et al., 2013). The outcome of this cell fusion is thus a transient hexaploid mitotic cell, with three maternal and three paternal sets of chromosomes (“3+3”), whereas its digynic germline counterpart comprises four maternal and two paternal sets (“2+2+2”). Although such a somatically fused cell is per definition tripolar, in terms of functionality it actually remains bipolar, since only the two centrosomes that derive from the mitotic cell are the operational active ones. As in the dyginic triploid zygote, this would in principle allow an appropriate reallocation of two complete triploid chromosome sets into daughter cells. However, such pure stable triploid cell populations can hardly be achieved, because fusions of somatic cells will always involve two already at least minimally differentiated cells with unequal epigenetic, metabolic and functional properties. Apart from the essential mitotic environment, the creation of viable progeny from such a fusion requires therefore the participation of two reasonably suited cell types that already contain and are able to contribute appropriate combinations of compatible and functionally interacting chromosomes to their offspring. Through this process, an appropriately fitted donor cell could likewise deliver fresh mitochondria to an energetically impaired mitotic cell and thereby increase the survival chances of the ensuing hybrid.