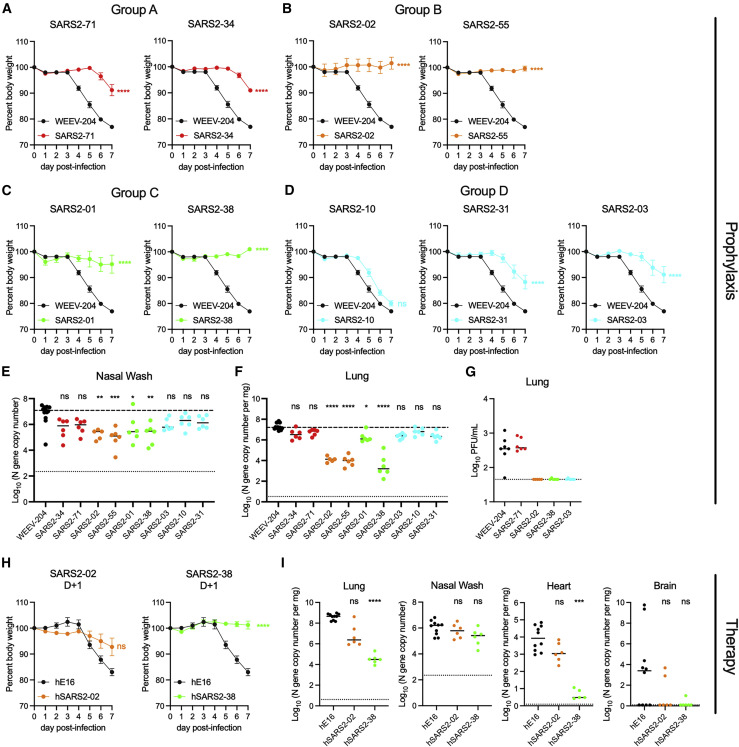
Figure 4.
Anti-SARS-CoV-2 mAbs protect against SARS-CoV-2 infection in vivo
(A–G) K18-hACE2 mice were passively administered 100 μg (~5 mg/kg) of the indicated mAb by intraperitoneal injection 24 h prior to intranasal inoculation with 103 FFU of SARS-CoV-2 WA1/2020.
(A–D) Mice were monitored for weight change for 7 days following viral infection. Mean weight change is shown. Error bars represent SEM.
(E and F) At 7 dpi, nasal washes (E) and lungs (F) were collected, and viral RNA levels were determined. Median levels are shown. The top dotted line indicates the median viral load of control mAb-treated mice. The bottom dotted line represents the limit of detection (LOD) of the assay.
(H) A subset of the lungs from (F) were assessed for infectious viral burden by plaque assay. Median PFU/mL is shown. The dotted line indicates the LOD.
(H and I) K18-hACE2 mice were given 200 μg (~10 mg/kg) of the indicated mAb by intraperitoneal injection 24 h after intranasal inoculation with 102 FFU of SARS-CoV-2 WA1/2020. Data are from two or three experiments; WEEV-204 (isotype control): n = 10; SARS2-02 and SARS2-38: n = 6 per group.
(H) Mean weight change. Error bars represent SEM.
(I) At 7 dpi, lungs, nasal washes, heart, and brain were collected, and viral RNA levels were determined. Median levels are shown.
In (A)–(F), data for each mAb are from two experiments; WEEV-204 (isotype control): n = 12; all other mAbs: n = 5–6 per group. In (A)–(D) and (H), we used one-way ANOVA with Dunnett’s post-test of the area under the curve; ∗∗∗∗p < 0.0001. In (E), (F), and (I), we used a Kruskal-Wallis with Dunn’s post-test.; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.