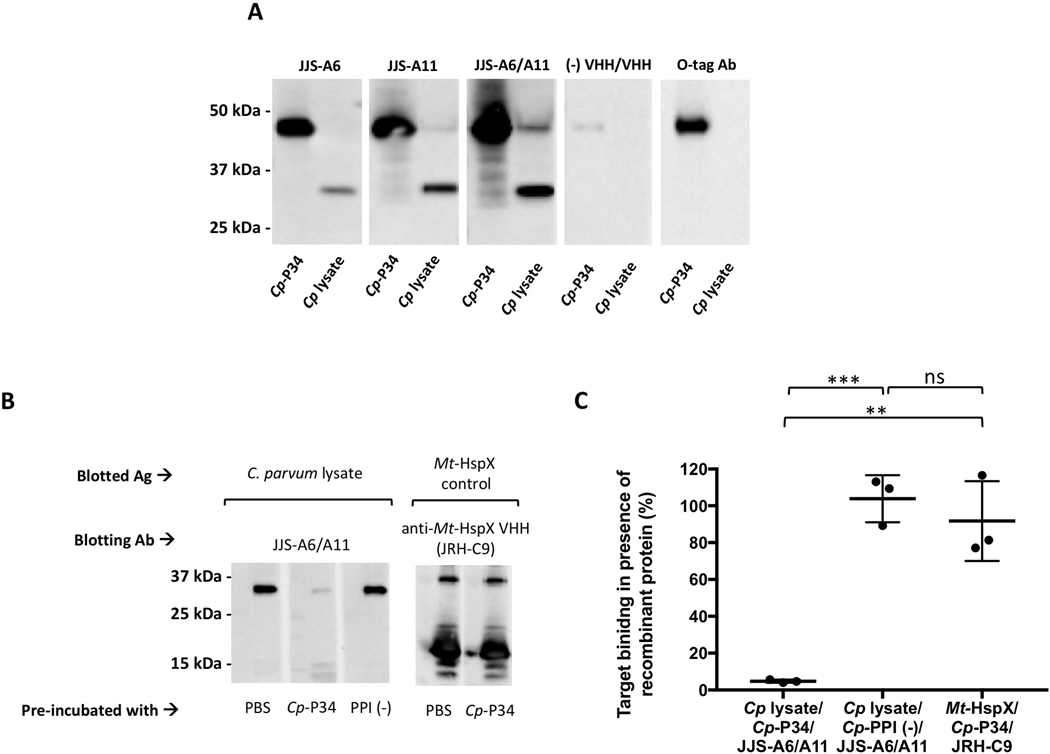
Fig. 3.
Identification of the ~34 kDa protein target as P34 of Cryptosporidium parvum (Cp-P34). (A) Recombinant Cp-P34 was resolved by SDS-PAGE in parallel with C. parvum lysate and blotted for recognition by the two JJS-A6 or JJS-A11 monomeric single-domain camelid antibodies (VHHs), the JJS-A6/A11 VHH dimer or a negative control VHH dimer control. The recombinant Cp-P34 is identified using an anti-OLLAS-tag horseradish peroxidase (HRP) conjugate. Ab, antibody (B) Cryptosporidium parvum lysate was separated and blotted with the mixture of a VHH at 0.01 μg/mL (0.2 nM for dimers and 0.3 nM for monomers) with recombinant protein at 0.25 μg/mL or PBS. A recombinant heat-shock protein of Mycobacterium tuberculosis (Mt-HspX) was included as a control antigen and blotted with an anti-HspX VHH monomer (JRH-C9). Additionally, recombinant peptidylprolyl isomerase of C. parvum (PPI) was included as a non-specific recombinant protein control. Ag, antigen (C) Summary of densitometry analysis from B indicating the percent of VHH binding in the presence of recombinant proteins as standardized to the PBS control. The binding of a JJS-A6/A11 heterodimer was blocked in the presence of recombinant Cp-P34 by 95.2±0.8% (t-test, **P=0.002, ***P=0.0002) but was unaffected in presence of the non-specific negative control protein Cp-PPI (t-test, P=0.44). Additional control indicates that JRH-C9 binding to Mt-HspX was unaffected by the presence of the recombinant Cp-P34 and maintained its binding at 91.7±21.6%. Values indicate means and error bars indicate S.D. (n=3). ns, not significant.