Figure 4.
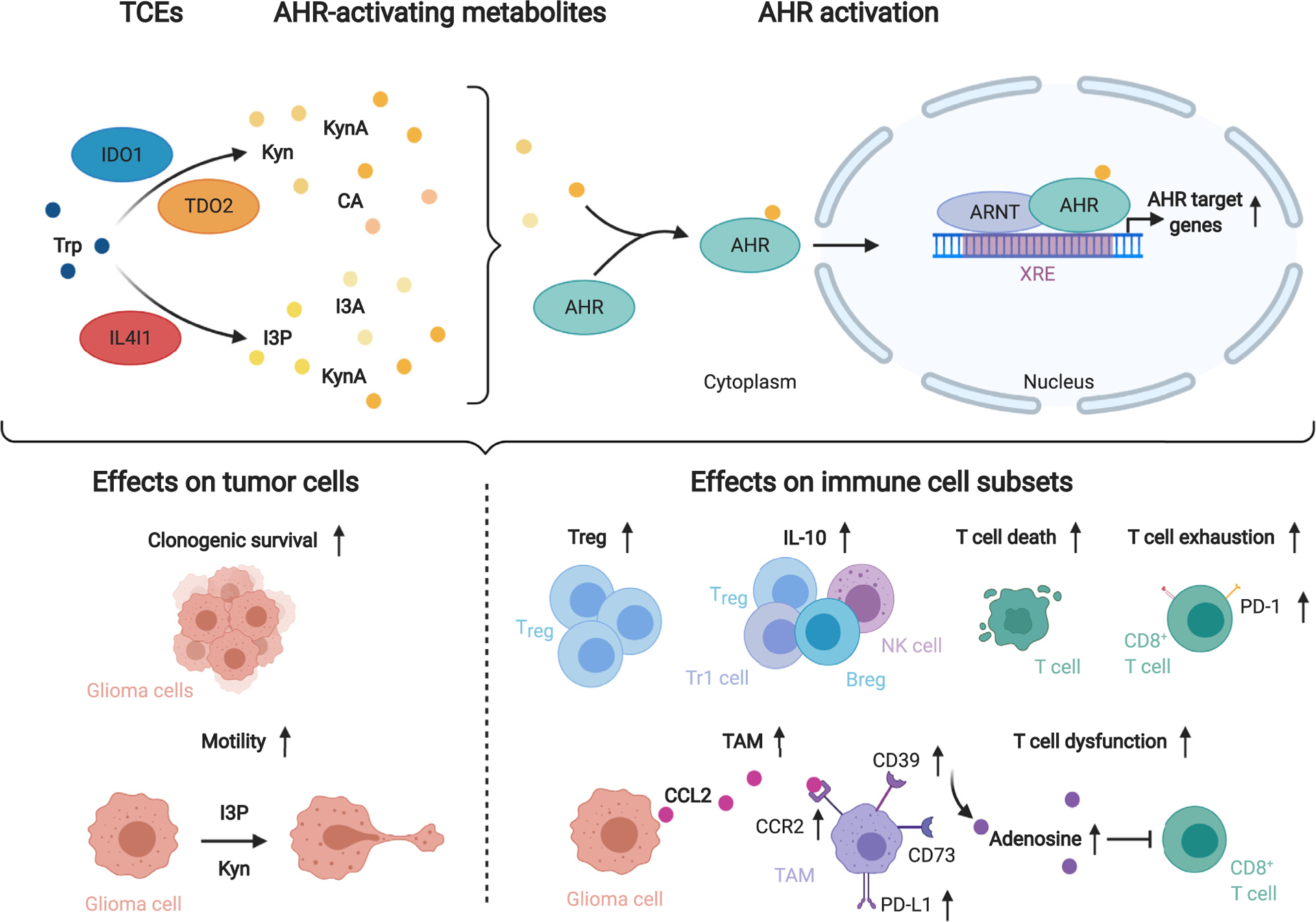
Tryptophan catabolism promotes tumor cell malignancy and immune suppression via activation of the aryl hydrocarbon receptor (AHR).
The tryptophan (Trp)-catabolic enzymes (TCEs) indoleamine-2,3-dioxygenase 1 (IDO1) and tryptophan-2,3-dioxygenase 2 (TDO2) initiate Trp degradation to the AHR-activating metabolites kynurenine (Kyn), kynurenic acid (KynA) and cinnabarinic acid (CA), while interleukin-4-induced-1 (IL4I1) generates the AHR agonists indole-3-pyruvic acid (I3P), indole-3-aldehyde (I3A) and KynA. Upon ligand binding the AHR translocates to the nucleus and binds to AHR nuclear translocator (ARNT). The heterodimer binds to xenobiotic response elements (XRE) in the promoters of AHR target genes increasing their expression. AHR activation enhances glioma cell malignancy. Furthermore, several immune cell subsets, including CD8+ T cells, regulatory T cells (Treg), Type 1 regulatory T cells (Tr1), regulatory B cells (Breg), natural killer (NK) cells and tumor-associated macrophages (TAM) are influenced by AHR activation. TAMs express the ectonucleotidases CD39 and CD73 that collaboratively produce adenosine which limit T cell function. Abbreviations: Interleukin (IL)-10, Programmed cell death protein-1 (PD-1), PD-ligand 1 (PD-L1), C-C motif chemokine receptor 2 (CCR2), C-C motif chemokine ligand 2 (CCL2).
