Table 1.
Further reaction optimizationa
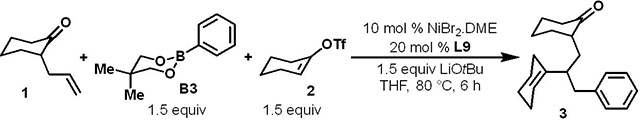
| ||
|---|---|---|
|
| ||
| entry | deviation in reaction condition | yields of 3 (%)b |
|
| ||
| 1 | none | 75 (72)c |
| 2 | Lithium-1-adamantanolate (LiOAd) instead of LiOtBu | 75 |
| 3 | LiOMe instead of LiOtBu | 23 |
| 4 | LiOiPr instead of LiOtBu | 15 |
| 5 | 1 equiv LiOtBu instead of 1.5 equiv | 42 |
| 6 | 2 equiv LiOtBu instead of 1.5 equiv | 65 |
| 7 | NiI2 or NiCl2·DME instead of NiBr2.DME | 70–72 |
| 8 | Ni(TMHD)2 instead of NiBr2·DME | 53 |
| 9 | Ni(PPh3)4 instead of NiBr2·DME | 12 |
| 10 | Ni(cod)2 instead of NiBr2·DME | 52 |
| 11 | other metals instead of NiBr2·DMEd | 0 |
| 12 | room temperature instead of 80 °C | 10 |
| 13 | 60 °C instead of 80 °C | 62 |
| 14 | 2-Me-THF instead of THF | 70 |
| 15 | 1,4-dioxane instead of THF | 60 |
| 16 | MeCN or toluene instead of THF | 35–38 |
| 17 | NMP, DMF, DMSO or dioxane instead of THF | 0–25 |
Reactions run at 0.10 mmol scale in 0.5 mL solvent.
1H NMR yields with pyrene as a standard.
The yield of isolated product from a 0.50 mmol scale reaction in 2.5 mL THF in parenthesis. dr is 1.1:1.
Pd(OAc)2, CuI, Co(OAc)2, FeCl2.
