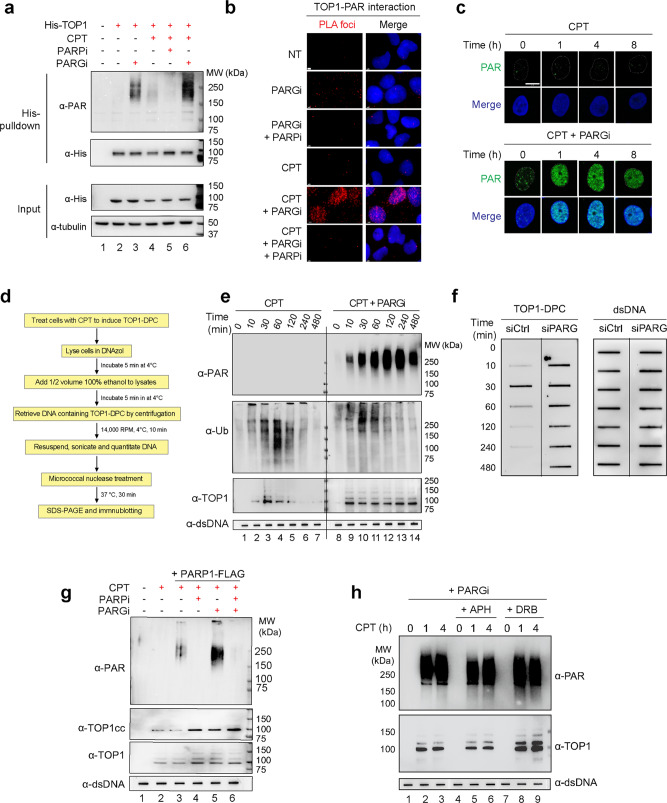
Fig. 2. Inhibiting PARG reveals the otherwise transient PARylation of TOP1-DPC and stabilizes TOP1-DPCs.
a His-tag pulldown assay showing reversible PARylation of transfected His-TOP1. Following transfection of 6×His-tagged TOP1 expression construct, HEK293 cells were treated with the indicated drugs for His-tag pulldown using Ni-NTA agarose under denaturing conditions. The pulldown samples and input samples were subjected to IB using α-TOP1 and α-PAR antibodies. CPT: 20 µM, 30 min; PARPi: 10 µM, 1 h pre-treatment; PARGi: 10 µM, 1 h pre-treatment. b Proximity ligation assay (PLA) showing TOP1-PAR interaction in cells treated with CPT and PARGi. Following transfection of 6×His-tagged TOP1 expression construct, U2OS cells were treated with the indicated drugs for PLA using rabbit α-His-tag antibody and mouse α-PAR antibody (10H). The scale bar represents 3 μm. c PARGi revealed CPT-induced PAR polymers on chromatin. After pre-extraction, U2OS cells treated as indicated were subjected to IF using α-PAR antibody (10H). CPT: 20 µM; PARGi: 10 µM, 1 h pre-treatment. The scale bar represents 10 μm. d Scheme of immunodetection of TOP1-DPC PARylation in vivo by modification of RADAR assay. TOP1-DPC PARylation was detected with α-PAR antibody (10H). e PARGi induced the hyper-PARylation and stabilization of TOP1-DPCs. HEK293 cells were treated with CPT (20 µM) in the absence or presence of PARGi (10 µM, 1 h pre-treatment). Cells were collected at the indicated time points for the modified RADAR for detection of TOP1-DPCs and their PARylation and ubiquitylation using α-TOP1, α-PAR, and α-Ub antibodies. 2 µg of digested DNA from each sample was subjected for slot-blotting using α-dsDNA antibody as a loading control. f ICE assay confirming that PARG deficiency blocked the removal of TOP1-DPCs. HEK293 cells transfected with control siRNA (siCtrl) or siRNA targeting PARG were treated with CPT (2 µM). Cells were collected at the indicated time points for ICE assay. 2 µg of digested DNA from each ICE assay sample was subjected for slot-blotting using α-TOP1 antibody or α-dsDNA antibody. g The modified RADAR assay showing that in vivo TOP1-DPC PARylation by PARP1 was counteracted by PARG. After transfection of the PARP1-FLAG expression plasmid, HEK293 cells were pre-treated with PARPi (10 µM, 1 h), PARGi (10 µM, 1 h), or PARPi + PARGi, followed by CPT treatment (20 µM, 1 h). The cells were then subjected to the modified RADAR assay for detection of TOP1-DPCs and their PARylation using α-TOP1 and α-PAR antibodies. h Inhibiting replication or transcription did not affect TOP1-DPC PARylation. HEK263 cells were pre-treated PARGi (10 µM, 1 h) in the absence or presence of the replication inhibitor aphidicolin (APH, 10 µM, 1 h) or the transcription inhibitor DRB (100 µM, 1 h). Cells were co-treated with 20 µM CPT for 0, 1, or 4 h, followed by detection of TOP1-DPCs and their PARylation by the modified RADAR assay using α-TOP1 and α-PAR antibodies.