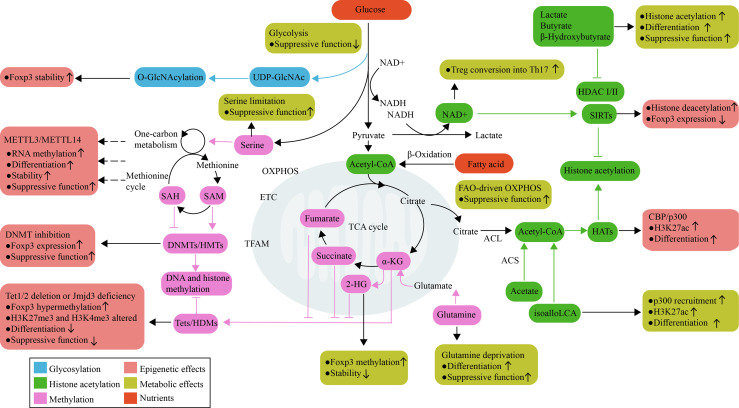
Figure 2.
Metabolic control of epigenetics in Treg cells. Treg cell activation, differentiation, and function are linked to metabolic reprogramming. Metabolic pathways not only process nutrients to produce ATP and meet energy requirements, but also use available metabolites as subtracts and cofactors for epigenetic enzymes that control Treg cell development. Glucose and glutamine are used to fuel O-GlcNAcylation, which can stabilize Foxp3. The mitochondria-derived metabolites α-KG, 2-HG, succinate, and fumarate are important for the function of DNA and histone demethylases; their effects oppose the actions of HMTs and DNMTs, which are regulated by amino acids that initiate one-carbon metabolism pathways and the methionine cycle. Histone acetylation is dependent on the supply of acetyl-CoA, which can be generated through a range of metabolic pathways. The product of bacterial anaerobic fermentation butyrate, as an inhibitor of HDACs, increases histone H3 acetylation in the Foxp3 locus and promotes Treg cell function. The SIRT family of enzymes promotes deacetylation function, which is dependent on NAD+ availability as a cofactor and regulated by the NAD+/NADH ratio. RNA methylation also requires the transfer of a methyl group; however, the contribution of metabolites to RNA methylation has not yet been explored in Treg cells. 2-HG, 2-hydroxyglutarate; α-KG, α-ketoglutarate; ACL, ATP-citrate lyase; ACS, acetyl-CoA synthetase; DNMTs, DNA methyltransferases; ETC, electron transport chain; GlcNAc, N-acetylglucosamine; HATs, Histone acetyltransferase; HDMs, histone demethylases; HMTs, histone methyltransferases; OXPHOS, oxidative phosphorylation; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SCFAs, short-chain fatty acids; SIRT, sirtuin; TCA, tricarboxylic acid; Tets, ten-eleven translocation family members.