Abstract
Background
Seroprevalence of anti-SARS-CoV-2 antibodies is now available in several world regions to better estimate transmission dynamics. However, to date, there is no epidemiological data regarding anti-SARS-CoV-2 prevalence in Mexico. Therefore, we aimed to determine the prevalence of anti-SARS-CoV-2 antibodies and define the clinical and demographic characteristics associated with seroprevalence.
Methods
We conducted a cross-sectional serological survey in Ciudad Guadalupe, NL, Mexico. City government employees voluntarily participated during July 2020. Demographic and clinical characteristics were collected at the time of blood sampling to analyze the associated characteristics. IgM/IgG antibodies were determined using a qualitative chemiluminescent immunoassay. Descriptive statistics were used for categorical and continuous variables. Statistical significance was tested using the Chi-squared test, Student’s t-test and the Mann–Whitney. Logistic regression models and the odds ratios (adjusted and unadjusted) were used to estimate the association of demographic and clinical characteristics.
Results
Of the 3,268 participants included, 193 (5.9%, 95% CI 5.1–6.8) tested positive for IgM/IgG against SARS-CoV-2. Sex, city of residence, and comorbidities did not show any association with having IgM/IgG antibodies. A total of 114 out of 193 (59.1%) subjects with a positive test were asymptomatic, and the odds of being positive were higher in those who reported symptoms of COVID-19 in the previous four weeks to the survey (OR 4.1, 95% CI 2.9–5.5).
Conclusions
There is a low rate of SARS-CoV-2 infection among government employees that have continuously been working during the pandemic. Six in ten infections were asymptomatic, and seroprevalence is low and still far from herd immunity. Epidemiological surveillance and preventive measures should be mandatory.
Keywords: COVID-19, Prevalence, Survey, Serology, Anti-SARS-CoV-2, Mexico
Background
Since the first case reported of the SARS-CoV-2 virus was reported in Mexico (February 27, 2020) to date (August 30th, 2020), there are 595,841 confirmed cases and 64,158 deaths [1]. Nuevo Leon, a northern state comprising a population of 5,119,504 inhabitants and which includes one out of the three most populated metropolitan areas in Mexico, has registered 49,457 confirmed cases and 2,403 deaths (August 30th, 2020), placing it in the third position nationwide [2]. Despite the increasing epidemiological surveillance efforts using the gold-standard quantitative polymerase chain reaction (PCR), testing is limited to symptomatic individuals, and it varies widely according to the test availability, even in the same country. Moreover, the occurrence of mild or asymptomatic infections leads to an underestimation of cases. Thus, seroepidemiological studies will allow better estimates of transmission dynamics, cumulative prevalence, and the proportion of the remaining susceptible populations [3].
Seroprevalence of anti-SARS-CoV-2 antibodies is now available in several world regions. In Europe, firstly affected by SARS-CoV-2, Germany, Spain, and Switzerland determined a 1.22% (March–May 2020), 4.6% (May 2020), and 4.8% (April–May 2020) antibody prevalence, respectively [4–6]. In the Americas, a 2.8% seroprevalence was reported in the state of California, United States, during April 2020, while 0.22% was observed in Brazil during May 2020 in a population-based survey [7, 8]. However, these serological surveys have been conducted at different epidemic phases in each country, using several immunological assays, sample sizes, and study designs in selected populations (population-based, blood donors, health-care workers) [5, 9, 10]. Thus, information derived from other regions is not a true reflection of the local epidemic's occurrence and transmission. To date, there is no epidemiological data regarding anti-SARS-CoV-2 prevalence in Mexico.
We conducted a cross-sectional serological survey in a population of government employees for the qualitative detection of IgM and IgG antibodies against SARS-CoV-2. We aimed to determine, as a primary endpoint, the prevalence of anti-SARS-CoV-2 antibodies and as a secondary endpoint, we defined the clinical and demographic characteristics associated with seroprevalence in the studied population. These results are now implemented for pandemic management in our regional context.
Methods
Study design and participants
We conducted a community level serological survey in Ciudad Guadalupe, the second most populated city (682,880 inhabitants) included in the metropolitan area of Monterrey, Nuevo Leon, Mexico [11]. The participants were drawn from a complete database of 4,220 government employees who were voluntarily invited to participate in this serological survey. Participants were included from July 9 to July 23, 2020. The work was carried out in accordance with Declaration of Helsinki for studies involving humans. Written informed consent was obtained from all participants, according to the research protocol approved by the Institutional Review Board of the School of Medicine and “Dr. Jose E. Gonzalez” University Hospital. Fieldwork was carried out by trained health care professionals in the community health clinic. We obtained demographic (age, sex, zip code, hometown, occupation) and clinical information using a standardized electronic questionnaire focused on symptoms related to coronavirus disease 19 (COVID-19), contact with confirmed cases, and comorbidities. After a 4 h fasting period, blood samples were collected, labeled, and sent to the Metabolic Research Laboratory at the Endocrinology Division of the “Dr. Jose Eleuterio Gonzalez” University Hospital.
Detection of anti-SARS-CoV-2 antibodies
We selected a qualitative chemiluminescent immunoassay to detect IgM/IgG against SARS-CoV-2 (Elecsys Anti-SARS-CoV-2, Roche, Germany, Ref. 09203079190) using the Cobas e801 automated analyzer (Roche, Germany). The National Health Department approved this test for the diagnostic intended use. The manufacturer reported a 99.5% sensitivity and specificity of 99.8% after 14 days post-PCR confirmation. This test uses a recombinant protein representing the nucleocapsid (N) antigen for the determination of antibodies against SARS‐CoV‐2 in the serum sample. The software automatically determines chemiluminescent emission by comparing the electrochemiluminescence signal obtained from the sample's reaction product with the signal of the cut-off value previously obtained by calibration (COI, cut-off index) using the positive and negative quality controls.
Statistical analysis
Statistical analysis was performed in the IBM SPSS Statistic software V22 (IBM Corp., USA) and EpiR for the Statistical Analysis of Epidemiology package [12]. The sample size calculated considering a SARS-CoV-2 seroprevalence of 5%, with a precision of ± 2.0% at the 95% confidence intervals, was 608 subjects. We reported the raw frequencies of positive tests as a proportion of the sample size. We compared our estimated cumulative prevalence against the official government PCR-based data of confirmed cases at the time of the survey. Descriptive statistics were used for categorical (frequencies and percentages) and continuous (means and standard deviations) variables. Statistical significance (P-value < 0.05) was tested using the Chi-squared and Fisher test for categorical variables, while Student’s t-test and the Mann–Whitney test were performed for continuous variables. Logistic regression models and the odds ratios (adjusted and unadjusted) were used to estimate the association of demographic and clinical characteristics identified as statistically significant in the bivariate analysis.
Results
Overall study population
A total of 4220 subjects were invited to participate in the serological survey, including 694 retired employees. During the 11 days of fieldwork, we collected 3268 (77.4%) blood samples and clinical data and 952 (22.6%) subjects did not attend the invitation due to planned vacations, sick leave, and work schedule unavailability. Table 1 shows the demographic and clinical data. The median age was 40 years (IQR 31–49), and 61.4% were males. Median BMI was 28.4 kg/m2, and obesity and overweight were observed in 38 and 39.4% of the population, respectively. Previously known hypertension and type 2 diabetes were referred by 8.8% of participants for both diseases. Besides, 60.3% declared to live in Ciudad Guadalupe, and police, firefighters, public security, and administrative tasks were the main occupations registered.
Table 1.
Demographic and clinical characteristics of the population
| Total sample (n = 3268) | Negative IgM/IgG (n = 3075) | Positive IgM/IgG (n = 193) | P | |
|---|---|---|---|---|
| Males, n (%) | 2008 (61.4) | 1891 (61.5) | 117 (60.6) | 0.82 |
| Age, yrs | 0.29 | |||
| 18–34, n (%) | 1127 (34.5) | 1059 (34.5) | 68 (35.2) | |
| 35–49, n (%) | 1328 (40.6) | 1242 (40.4) | 86 (44.6) | |
| 50–65, n (%) | 765 (23.4) | 730 (23.7) | 35 (18.1) | |
| > 65, n (%) | 48 (1.5) | 44 (1.4) | 4 (2.1) | |
| Weighta, kg | 80.0 (69–90) | 80.0 (69–90) | 82 (72–92) | 0.13 |
| Heighta, m | 1.67 (1.6–1.7) | 1.67 (1.6–1.7) | 1.67 (1.6–1.7) | 0.86 |
| BMIa, kg/m2 | 28.41 (25.3–32) | 28.4 (25.2–32) | 29.0 (25.6–32.6) | 0.18 |
| BMI categories | 0.08 | |||
| Underweight, n (%) | 17 (0.5) | 17 (0.6) | 0 | |
| Normal, n (%) | 721 (22.1) | 685 (22.3) | 36 (18.7) | |
| Overweight, n (%) | 1289 (39.4) | 1209 (39.3) | 80 (41.5) | |
| Obesity I-III, n (%) | 1241 (38) | 1164 (37.8) | 77 (39.8) | |
| Comorbidities | ||||
| Hypertension, n (%) | 287 (8.8) | 269 (8.7) | 18 (9.3) | 0.79 |
| Diabetes type 2, n (%) | 287 (8.8) | 273 (8.9) | 14 (7.3) | 0.51 |
| Obesity, n (%) | 1241 (38) | 1164 (37.8) | 77 (39.8) | 0.59 |
| City | 0.08 | |||
| Guadalupe, n (%) | 1970 (60.3) | 1846 (60.0) | 124 (64.2) | |
| Monterrey, n (%) | 495 (15.1) | 257 (8.4) | 17 (8.8) | |
| Juárez, n (%) | 274 (8.4) | 465 (15.1) | 30 (15.5) | |
| Other, n (%) | 530 (16.2) | 507 (16.5) | 22 (11.5) | |
| Occupation | 0.09 | |||
| Police, firefighters and public safety, n (%) | 1051 (32.2) | 982 (31.9) | 69 (35.7) | |
| Office/Management, n (%) | 696 (21.2) | 660 (21.5) | 36 (18.7) | |
| Maintainance/Janitors, n (%) | 608 (18.7) | 583 (18.9) | 25 (12.9) | |
| Healt-care workers, n (%) | 163 (5) | 155 (5) | 8 (4.1) | |
| Other, n (%) | 750 (22.9) | 695 (22.7) | 55 (28.6) | |
aMedian (IQR). IgG Type G immunoglobulin, IgM Type M immunoglobulin
Prevalence of anti-SARS-CoV-2 and characteristics of seropositive subjects
We were able to identify anti-SARS-CoV-2 antibodies in 193 out of 3,268 subjects, resulting in a raw prevalence of 5.9% (95% CI 5.1–6.7) during July 2020. Cases with positive IgM/IgG were not different in age, sex, weight, height, or BMI from seronegative subjects. There was a statistical difference in the occupation category of cleaning/maintenance (P = 0.04). At the time of the survey, 83.9% of the participants had not experienced COVID-19 symptoms, while 33.7% of the subjects reported a known contact with a confirmed COVID-19 case. As expected, self-reported symptoms during the previous four weeks to the survey were statistically different between the groups (Table 2), including the contact with a confirmed COVID-19 case (P = 0.013).
Table 2.
COVID-19 symptoms during the previous four weeks at the time of the serosurvey
| Seronegative IgM/IgG (n = 3075) | Seropositive IgM/IgG (n = 193) | P | |
|---|---|---|---|
| Asymptomatic, n (%) | 2627 (85.4) | 114 (59.1) | < 0.001 |
| Symptomatic, n (%) | 448 (14.6) | 79 (40.9) | < 0.001 |
| Self-reported symptoms | |||
| Cough, n (%) | 119 (3.9) | 47 (24.4) | < 0.001 |
| Sore throat, n (%) | 202 (6.6) | 33 (17.1) | < 0.001 |
| Nasal discharge, n (%) | 85 (2.8) | 15 (7.8) | 0.001 |
| Headache, n (%) | 173 (5.6) | 32 (16.6) | < 0.001 |
| Anosmia, n (%) | 63 (2.0) | 40 (20.7) | < 0.001 |
| Muscle/body aches, n (%) | 98 (3.2) | 31 (16.1) | < 0.001 |
| Diarrhea, n (%) | 92 (3.0) | 23 (11.9) | < 0.001 |
| Fever, n (%) | 62 (2.0) | 29 (15.0) | < 0.001 |
| Shortness of breath, n (%) | 40 (1.3) | 21 (10.9) | < 0.001 |
| Contact with confirmed case, n (%) | 416 (13.5) | 39 (20.2) | 0.013 |
COVID-19 Coronavirus disease 2019, IgG Type G immunoglobulin, IgM Type M immunoglobulin
Characteristics associated with anti-SARS-CoV-2 seroprevalence
Sex, city of residence, and comorbidities did not show any association with having IgM/IgG antibodies against SARS-CoV-2 (Table 3); while 59.1% (114 out of 193) individuals with a positive test were asymptomatic. The odds of being positive were higher in those who reported COVID-19 symptoms in four weeks before the survey (OR 4.06, 95% CI 2.99–5.51). Anosmia was the most strongly associated symptom with seropositivity (OR 12.62, 95% CI 8.22–19.37), while sore throat was the least (OR 2.93, 95% CI 1.96–4.38). Inversely, being asymptomatic resulted in a 75% less probability of having a positive IgM/IgG test. Multivariable logistic regression analysis revealed that the odds of being positive were higher in those patients with previous symptoms such as cough, anosmia, and fever (Fig. 1). The initial protective effect for the occupation of cleaning/maintenance was no longer observed when adjusted in the multivariate model (OR 0.71, 95% CI 0.46–1.10, P = 0.13). Police, firefighters, and public security did not show any association in the univariate or multivariate models.
Table 3.
Univariate and multivariate analysis of factors associated with positive anti-SARS-CoV-2 antibodies
| IgM/IgG | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| Negative | Positive | OR | 95% CI | P | OR | 95% CI | P | |
| Sex | ||||||||
| Males, n (%) | 1891 (61.5) | 117 (60.6) | 0.96 | 0.72–1.30 | 0.82 | |||
| City of residence | ||||||||
| Guadalupe, n (%) | 1846 (60.0) | 124 (64.2) | 1.20 | 0.89–1.62 | 0.26 | |||
| Occupation | ||||||||
| Police, firefighters & public safety, n (%) | 982 (31.9) | 69 (35.8) | 1.19 | 0.88–1.61 | 0.27 | |||
| Office/Management, n (%) | 660 (21.5) | 36 (18.7) | 0.84 | 0.58–1.21 | 0.41 | |||
| Cleaning/Mantainance, n (%) | 583 (18.9) | 25 (12.9) | 0.64 | 0.41–0.98 | 0.04 | 0.71b | 0.46–1.10 | 0.13 |
| Health-care workers, n (%) | 155 (5.0) | 8 (4.1) | 0.82 | 0.40–1.68 | 0.73 | |||
| Other, n (%) | 695 (22.7) | 55 (28.6) | 1.37 | 0.99–1.89 | 0.06 | |||
| Comorbidities | ||||||||
| Hypertension, n (%) | 269 (8.7) | 18 (9.3) | 1.07 | 0.65–1.77 | 0.79 | |||
| Diabetes, n (%) | 273 (8.9) | 14 (7.3) | 0.80 | 0.46–1.40 | 0.51 | |||
| Obesity, n (%) | 1164 (37.9) | 77 (39.9) | 1.09 | 0.81–1.47 | 0.59 | |||
| Any comorbiditiea, n (%) | 1481 (48.2) | 100 (51.8) | 1.16 | 0.87–1.55 | 0.34 | |||
| COVID-19 symptoms | ||||||||
| Asymptomatic, n (%) | 2627 (85.4) | 114 (59.1) | 0.25 | 0.18–0.33 | < 0.001 | |||
| Symptomatic, n (%) | 448 (14.6) | 79 (40.9) | 4.06 | 2.99–5.51 | < 0.001 | 3.91b | 2.86–5.35 | < 0.001 |
| Cough, n (%) | 119 (3.9) | 47 (24.4) | 7.98 | 5.49–11.65 | < 0.001 | 4.17c | 2.50–6.93 | < 0.001 |
| Sore throat, n (%) | 202 (6.6) | 33 (17.1) | 2.93 | 1.96–4.38 | < 0.001 | |||
| Nasal discharge, n (%) | 85 (2.8) | 15 (7.8) | 2.97 | 1.68–5.24 | 0.001 | |||
| Shortness of breath, n (%) | 40 (1.3) | 21 (10.9) | 9.26 | 5.34–16.06 | < 0.001 | |||
| Headache, n (%) | 173 (5.6) | 32 (16.6) | 3.33 | 2.21–5.02 | < 0.001 | |||
| Anosmia, n (%) | 63 (2.0) | 40 (20.7) | 12.62 | 8.22–19.37 | < 0.001 | 5.75c | 3.41–9.71 | < 0.001 |
| Diarrhea, n (%) | 92 (3.0) | 23 (11.9) | 4.39 | 2.71–7.12 | < 0.001 | |||
| Muscle pain, n (%) | 98 (3.2) | 31 (16.1) | 5.81 | 3.77–8.97 | < 0.001 | |||
| Fever, n (%) | 62 (2.0) | 29 (15.0) | 8.59 | 5.38–13.72 | < 0.001 | 2.44c | 1.30–4.58 | 0.006 |
| Contact with confirmed case, n (%) | 416 (13.5) | 39 (20.2) | 1.62 | 1.12–2.34 | 0.013 | |||
aOther cardiovascular, allergic, endocrine, and neurological diseases. bAdjusted for cleaning/maintenance, symptoms, and contact with a confirmed case. cAdjusted for contact with a confirmed case, cleaning/maintenance, sore throat, and headache. OR Odds ratio, CI Confidence interval
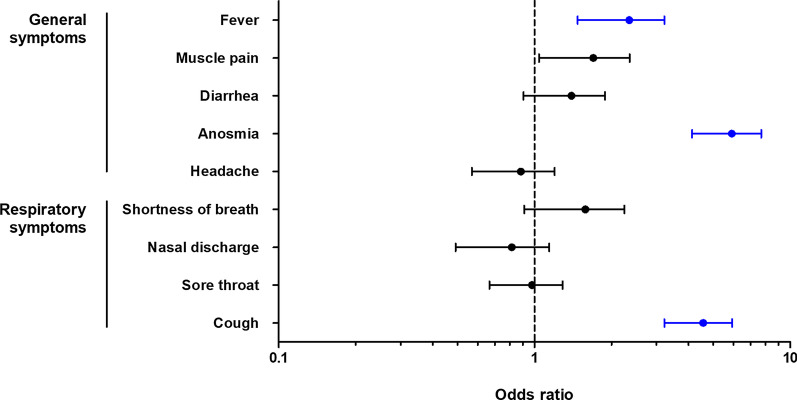
Fig. 1.
Odds ratios for symptoms associated with positive IgM/IgG against SARS-CoV-2 in the multivariate model. In blue, statistically significant symptoms: anosmia (5.4, 95% CI 3.1–9.2, P < 0.001), cough (4.2, 95% CI 2.4–7.1, P < 0.001), and fever (2.0, 95% CI 1.02–3.9, P = 0.04)
Discussion
Our findings from this regional cross-sectional seroprevalence study for SARS-CoV-2 indicate that the prevalence of IgM/IgG antibodies against the novel coronavirus is 5.9% in a city of the metropolitan area of Monterrey. This study is the first data available, since the first case registered in February 2020, on the prevalence of anti-SARS-CoV-2 antibodies in our country. Because our survey was designed to obtain information at a local level, regional differences in cultural habits and other factors might be observed against other areas in Mexico. As the third most populated state in the country, Monterrey's metropolitan area was considered a hotspot for epidemic transmission, and our findings may provide additional data to complete epidemiological surveillance.
Several studies have been published since April 2020 regarding the prevalence of anti-SARS-CoV-2 antibodies. Each one provided a snapshot that mirrors time and space-specific circumstances in the different studied populations and the associated characteristics. Our prevalence was higher than those reported in California, United States (April 2020), and Spain and Switzerland during last May 2020 [5, 6], but lower than the 7.3% determined in the 1104 samples from the general population of Stockholm, Sweden at the end of April, three months after the pandemic arrival [13]. Previous data for other regions or states in Mexico are not currently available. Given the asynchrony in COVID-19 transmission in our country, different seroprevalences might be found. Serosurveys are a useful tool to determine the previous exposure to SARS-CoV-2 and better estimate the true number of infections with and without symptoms. Particularly, Mexico has very limited PCR testing based on the sentinel epidemiological model used for epidemiological surveillance. This survey included a large sample, allowing determining an initial reference of previous exposure in asymptomatic individuals. Furthermore, sampling was made in a selected population that belongs to the exposed essential government workforce. Also, demographic characteristics such as age, sex, and city of residence of the sampled population were different from the overall population and may affect generalizability of the results. These characteristics will make it feasible for prospective and follow-up studies. In conjunction, the prevalence of 5.9%, implying that around 40,289 people were infected in Ciudad Guadalupe (population 682,880 in 2015) during July 2020 [11], 10.3 times the average 3,898 PCR confirmed cases at the time of this survey (July 23, 2020) [2]. Notably, Nuevo Leon has the higher rate of PCR test per million people in our country. Similar assumptions could be made for other regions as soon as local data become available.
In agreement with our findings, sex was not associated with anti-SARS-CoV-2 antibodies either in selected populations, health-care workers, and population-based studies. In contrast, age was associated with higher odds of having a positive test in Brazilian blood donors [14], while the opposite was seen in Swiss children and adults > 65 years in a population-based survey [6]. Consistently with our results, no association was observed in the professional category, previous contact with a COVID-19 case, and comorbidities [15]. Although hypertension and type 2 diabetes were associated with a higher risk for poor outcomes in COVID-19 patients [16], no relationship was found with positive IgM/IgG. Educational level, household size, and symptoms were associated with the presence of antibodies [6, 14, 15]. In our study, as expected, previous COVID-19 symptoms were associated with seropositivity (OR 4.06, 95% CI 2.99–5.51, P < 0.001), although a higher OR was reported by Garcia-Bateiro et al. (OR 8.8, 95% CI 4.41–17.73, P < 0.0001) [15]. Among the symptoms reported, anosmia showed the strongest association in our analysis. Asymptomatic cases (59.1%) compared with other countries were higher than the 32.7%, and 52.4% reported in the general population and health-care workers in Spain, respectively [5, 15]. As much as approximately 80% of infections are expected to be asymptomatic or mild [17].
Recent studies indicate some limitations of antibody testing, besides the inherent diagnostic performance. Although antibodies are expected to correlate with infection and disease presentation, a recent Chinese study showed that some infected individuals did not produce neutralizing humoral evidence of SARS-CoV-2 infection. In 10 (5.7%) out of 175 COVID-19 recovered patients who experienced mild symptoms, neutralizing antibodies against SARS-CoV-2 were unable to cross-react with the SARS-CoV-2 virus [18]. Thus, other immune responses, such as T cells and cytokines, may be involved in recovery. Furthermore, Long et al. reported than 40% of asymptomatic patients became seronegative for IgG in the early convalescent phase [19] suggesting a weaker humoral response that might have implications in the results of serological surveys confounding the true exposure rate.
Our serosurvey has some limitations that may be considered. First, we performed an isolated IgM/IgG test, obtaining a cross-sectional seroprevalence. A prospective and longitudinal survey will be highly valuable in following seroconversion and determining household transmission and lockdown effect in the long-term. Second, children were not included in the survey; additional studies will be needed to determine SARS-CoV-2 spread in this group. Finally, due to the study design, these results represent our sampled population's prevalence estimates and do not necessarily reflect the entire state prevalence. Otherwise, as strengths for the study, we used an appropriate and powered sample size to provide a reference prevalence value. Also, it seems we surveyed at the right epidemic moment (WHO Phase 3). Besides, we used a high-sensitivity chemiluminescent immunoassay, as reported by Muench et al. [20], which has shown a better diagnostic performance than other tests.
Conclusions
In conclusion, this study provides a regional estimation of SARS-CoV-2 dissemination in the densely populated northern region of our country. Six in ten infections were asymptomatic, and despite the significant impact of COVID-19 in Mexico, seroprevalence is still very low and, more importantly, far from herd immunity. Epidemiological surveillance and preventive measures to avoid transmission, such as social distancing and facial masks use, should still be enforced in the population.
Acknowledgements
We thank Sergio Lozano-Rodríguez, M.D. for his critical reading and editing of the manuscript.
Abbreviations
- Anti-SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2 antibodies
- BMI
Body mass index
- CI
Confidence interval
- COI
Cut-off index
- COVID-19
Coronavirus disease 19
- IgM
Immunoglubulin type M
- IgG
Immunoglobulin type G
- PCR
Polymerase chain reaction
- OR
Odds ratio
Authors’ contribution
All named authors met the International Committee of Medical Journal Editors (ICMJE) criteria for authorship. CDS, ASG, and JGGG developed the concept and design. JGGG, CDS, and DSR contribute to the implementation of the study protocol. ASG, RRG, and ACO participated in data collection and statistical analysis. CDS, ASG, RRG, and JGGG contributed to data interpretation, and ASG, CDS, and ACO wrote the manuscript with input from all authors. All authors provided critical feedback for intellectual content and approved the final version of the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Endocrinology Division, Department of Internal Medicine of the “Dr. Jose Eleuterio Gonzalez” University Hospital, Universidad Autónoma de Nuevo León.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the Academic Digital Repository (Univesidad Autónoma de Nuevo León), [http://eprints.uanl.mx/19978/].
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the School of Medicine and “Dr. Jose E. Gonzalez” University Hospital (EN20-00025). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cristina Díaz-Salazar and Adriana Sánchez-García contributed equally to this work
References
- 1.COVID-19 Mexico Database. National Epidemiology Center. Mexico Government. 2020. https://coronavirus.gob.mx/datos/#DOView. Accessed 31 Aug 2020.
- 2.COVID-19 Cases in Nuevo León. Nuevo León Health Department. 2020. https://www.nl.gob.mx/publicaciones/casos-de-covid-19-en-nuevo-leon. Accessed 31 Aug 2020.
- 3.Barrientos-Gutiérrez T, Alpuche-Aranda C, Lazcano-Ponce E, Pérez-Ferrer C, Rivera-Dommarco J. Public health in the first wave: a research agenda for cooperation under Covid-19. Salud Publica Mex. 2020;62(5):598–606. doi: 10.21149/11606. [DOI] [PubMed] [Google Scholar]
- 4.Fischer B, Knabbe C, Vollmer T. SARS-CoV-2 IgG seroprevalence in blood donors located in three different federal states, Germany, March to June 2020. Euro Surveill. 2020;25(58):2001285. doi: 10.2807/1560-7917.ES.2020.25.28.2001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollán M, Pérez-Gómez B, Partor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stringhini S, Wisniak A, Piumatii G, Azman A, Lauer SA, Baysson H, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendavid E, Mulaney B, Sood N, Shah S, Ling E, Bromley-Dulfano R, et al. COVID-19 antibody seroprevalence in Santa Clara county. California Int J Epimeiol. 2021;50(2):410–419. doi: 10.1093/ije/dyab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silveira MF, Barros AJD, Horta BL, Pellanda LC, Victora GD, Dellagostin OA, et al. Population-based surveys of antibodies against SARS-CoV-2 in Southern Brazil. Nat Med. 2020;26(8):1196–1199. doi: 10.1038/s41591-020-0992-3. [DOI] [PubMed] [Google Scholar]
- 9.Moscola J, Sembajwe G, Jarrett M, Farber B, Chang T, McGinn T, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu R, Huang J, Duan C, Liao Q, Shan Z, Wang M, et al. Low prevalence of antibodies against SARS-CoV-2 among voluntary blood donors in Guangzhou. China J Med Virol. 2020 doi: 10.1002/jmv.26445.10.1002/jmv.26445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Data Nuevo Leon. Nuevo Leon Economy Department. Nuevo Leon Government. 2020. http://datos.nl.gob.mx/n-l-poblacion-total-y-por-municipio/. Accessed 26 Aug 2020.
- 12.Bendix Carstensen, Martyn Plummer, Esa Laara, Michael Hills. Epi: a package for statistical analysis in epidemiology. R package version 1.1.44. 2013. http://CRAN.R-project.org/package=Epi. Accessed 8 Aug 2020.
- 13.Public Health Agency Sweden. Första resultaten från pågående undersökning av antikroppar för covid-19-virus. 2020. https://www.folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2020/maj/forsta-resultaten-fran-pagaende-undersokning-av-antikroppar-for-covid-19-virus. Accessed 12 Aug 2020.
- 14.Amorim-Filho L, Szwarcwald CL, Mateos S, Leon ACM, Medronho R, Veloso VG. Seroprevalence of IgG and IgM anti-SARS-CoV-2 among voluntary blood donors in Rio de Janeiro, Brazil. COVID-19 Global literature on coronavirus disease. Rev Saude Publica. 2020;54:69. doi: 10.11606/s1518-8787.2020054002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Basteiro AL, Moncunill G, Tortajada M, Vidal M, Guinocart C, Jiménez A, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11(1):3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrera FJ, Shekhar S, Wurth R, Moreno-Pena PJ, Ponce OJ, Hajdenberg M, et al. Prevalence of diabetes and hypertension and their associated risks for poor outcomes in Covid-19 patients. J Endocr Soc. 2020 doi: 10.1210/jendso/bvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckerle I, Meyer B. SARS-CoV-2 seroprevalence in COVID-19 hotspots. Lancet. 2020;396(10250):514–515. doi: 10.1016/S0140-6736(20)31482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu F, Wang A, Liu M, Wang Q, Chen J, Xia S, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. Medrxiv. 2020 doi: 10.1101/2020.03.30.20047365. [DOI] [Google Scholar]
- 19.Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 20.Muench P, Jochum S, Wenderoth V, Ofenloch-Haehnle B, Hombach M, Strobl A, et al. Development and validation of the elecsys Anti-SARS-CoV-2 immunoassay as a highly specific tool for determining past exposure to SARS-CoV-2. J Clin Microbiol. 2020;58(10):e01694–e1720. doi: 10.1128/JCM.01694-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available in the Academic Digital Repository (Univesidad Autónoma de Nuevo León), [http://eprints.uanl.mx/19978/].