Abstract
Implantable drug-delivery microdevices are a key diagnostic and therapeutic tool in medicine with increasing applications. Preparation of such combination drug-delivery devices for human studies requires the development of methods to ensure sterility, safety and integrity on both the device and drug side. Despite growing applications for these technologies, there has been a lack of clear methodology regarding sterilization and preparation to meet strict guidelines set forth by the Food and Drug Administration (FDA). Our laboratory developed a set of widely applicable and straightforward procedures to prepare drug-device combination products for clinical use that consistently achieve the high-quality standards provided by the FDA. This includes several newly developed methods for preparation of the implant including endotoxin removal, appropriate sterilization of raw materials, formulation of novel pharmaceutical agents, and loading of agents into drug delivery reservoirs. We also discuss protocols and methods developed with FDA to meet regulatory guidelines to ensure continual sterility and endotoxin testing, as well as longer-term stability testing of drugs and biologic agents.
-
•
Endotoxin removal and sterilization of raw materials for clinical use.
-
•
Formulation and device loading of novel pharmaceutical agents.
-
•
Continued testing of pharmaceutical agents and devices to meet regulatory guidelines.
Keywords: Biomedical engineering, Drug formulation, Sterilization, Medical device, Implants
Graphical abstract
Specification table
| Subject Area: | Medical Devices, Clinical Trials |
| More specific subject area: | Medical devices in clinical trials |
| Method name: | Preparation of an implantable device for clinical use |
| Name and reference of original method: | N/A |
| Resource availability: | Brigham and Women's Hospital, Dana-Farber Cancer Institute, Medical Device Academy |
Introduction
The major goal of the methods and protocols presented in this study is to provide a framework for first-in-human use of drug-device implants. The translational step from animal studies to humans represents a major hurdle for advancing biomedical innovations. The critical hurdle of this translational step is the regulatory safety review by FDA and Institutional Review Boards prior to human studies.
Our group previously developed an implantable microdevice (IMD) loaded with up to 20 different chemotherapeutics that is inserted percutaneously into tumors, and retrieved several hours to days later using standard-of-care methods with the goal of measuring the response of a given tumor to multiple therapies in order to identify optimal therapy on a personalized basis. [5]
Following proof-of-concept studies with these devices in animals, a major obstacle to further development of this platform was to meet strict FDA guidelines for human applications.
We describe the methods developed in conjunction with FDA to ensure safety and feasibility of the IMD with a focus on cleaning, sterilization and preparation of implants. The protocols were adapted for our platform, and many of them were newly developed. Although the specific implantable microdevice used in our trials is unique, these methods can be broadly applied to many other types of short-term and long-term medical implants for other researchers to follow.
Microdevice preparation
IMDs are manufactured on a 5-axis CNC micromachining station using subtractive machining techniques developed by our laboratory personnel. Full traceability of the stock material used for manufacturing is provided. In accordance with quality control guidelines, approximately 10% of each batch is randomly inspected upon arrival to ensure specifications for reservoir diameter, overall length, and overall diameter are met. A secondary manufacturing step involves attaching a nitinol guidewire to the IMD body using medical-grade epoxy (EPO-TEK MED-301) and a curing step according to the provided specifications.
Endotoxin and sterility steps
The FDA sets endotoxin and sterility limits for implants depending on the intended use of the device and the tissue which the device directly or indirectly contacts. Endotoxin limits vary based on the anatomical locations which the IMD can potentially contact. [2] Devices that directly or indirectly contact the cardiovascular system, which applies to most anatomical sites, have a limit of 20EU per patient. Devices that can potentially contact cerebrospinal fluid (including brain tumors) are subject to an endotoxin limit of 2.15 EU. If multiple implants are placed in a given patient, the EU limit per implant is calculated as the total EU limit per patient, divided by the number of devices implanted [2].
There is no published data identifying the most effective approach for endotoxin removal to consistently meet the FDA requirement of endotoxin limits below 20 EU per patient, or 4EU per device (assuming a total of up to 5 devices implanted per patient). Based on prior research, our initial attempt (“Method 1”) at endotoxin removal was conducted by preparing samples on a benchtop using sterile materials, followed by autoclaving with high-temperature dry heat and sterilizing with gamma irradiation, would meet endotoxin limits. [3] As shown in Fig. 1 method 1, preparation of samples using this method resulted in large deviations and overall unacceptable endotoxin limits.
Fig. 1.
Endotoxin values based on disinfection procedure. Several methods of endotoxin removal were hypothesized and tested prior to submitting a final procedure to the FDA. Method 1 resulted in the highest and least precise endotoxin values, whereas method 3 led to the lowest and most consistent values.
A second method of preparing samples using sterile materials within a biosafety cabinet (rather than on a benchtop), followed by autoclaving and gamma irradiation, was implemented. Test results demonstrate slightly reduced EU values with significantly reduced variability, but were nonetheless above tolerance limits for larger production lots in which the maximum individual value may define lot acceptance (Fig. 1, method 2).
A modified procedure (Method 3) was developed that added a 3-hour sodium hydroxide (NaOH) wash followed by rinsing with endotoxin-free water and autoclaving, prior to testing of endotoxin values. As seen in Fig. 1, Method 3 consistently reduced endotoxin limits below 1.00. We concluded that autoclaving with high-temperature dry heat combined with a base cleaning agent (NaOH) and gamma irradiation was the most effective endotoxin removal procedure. The following is the final procedure submitted as an appendix to FDA for clinical protocols:
-
1.
First, thoroughly spray all materials and packaging with 70% ethanol and place within a bio-safety cabinet.
-
2.
Place all materials to be sterilized into glass beakers in the bio-safety cabinet and fill the beakers with USP-grade Sodium Hydroxide (NaOH) solution, making sure to completely submerge the objects. Soak all materials for approximately 3 h to ensure maximum endotoxin removal.
-
3.
After 3 h, vacuum off the sodium hydroxide while under the biosafety cabinet, and rinse with endotoxin-free water.
-
4.
Repeat the rinsing step two more times, and autoclave the materials for a sterilization time of 30 min at 121o using a gravity cycle.
Drug preparation
All pharmaceutical agents used in our devices are purchased commercially through the Dana-Farber Cancer Institute (DFCI) Research Pharmacy and are FDA-approved for regular use in cancer patients. In drug delivery implants, these drugs are combined with poly-ethylene glycol (PEG) to form a drug-polymer mixture which is loaded into drug delivery reservoirs. Importantly, the FDA considers such drug-polymer mixtures new therapeutics for which stability and potency must be demonstrated.
Qualified personnel undertake the preparation, handling, and safe disposal of the chemotherapeutic agents in a self-contained and protected environment. Drugs are prepared, mixed with United States Pharmacopeia (USP)-grade PEG matrix, and loaded into the microdevices in a sterile manner in the research pharmacy. Preparation of drugs proceeds per the following protocol or with minor modifications:
-
1.
Weigh out the necessary amount of PEG on a microscale and put it in a sterile vial.
-
2.
Calculate amount of injection solution needed to achieve a 20% drug/80% PEG formulation. Use antiseptic technique to draw the appropriate amount of the injection vial, and add to the vial containing PEG. If it is an oral drug, use a mortar and pestle under a biosafety cabinet to break up the drug into powder form, add solvent to the container of the drug, and vortex until the solute dissolves completely.
-
3.
Evaporate the solvent on a rotary evaporator at 30 °C.
The drug formulation process occurs in an NSF/ANSI 49-certified biosafety cabinet, and all products are sprayed with 70% ethanol to maintain sterility and reduce endotoxin contamination. “Biologics” drugs will be used directly as a lyophilized powder without PEG addition or rotary evaporation. They are loaded into the microdevices after gamma irradiation.
Pharmaceutical steps
The next step in the IMD preparation process is loading IMDs with the formulated chemotherapy/polymer mixtures. This process is conducted in the research pharmacy clean room which is ISO 9001 certified, and the pharmacy in which the drugs are purchased from. IMDs are loaded in a multi-step process, which varies depending on whether large-molecule biologic agents are included. A Quality Assurance (QA) specialist documents the process.
If only small-molecule agents are used, the following directions apply:
-
1.
Reservoirs are loaded with all small-molecule agents.
-
2.
Loaded microdevices are placed inside a hollow biopsy needle, and bone wax is placed at the tip to prevent the devices from falling out. An inner stylet is pushed through the hollow biopsy needle until it touches the microdevice. It is then kept in place with a needle lock.
-
3.
Each needle assembly is placed into a sterilization pouch with a desiccant pouch.
-
4.
Pouches are sent to an outside contractor for gamma irradiation, followed by sterility and endotoxin testing.
If biological agents are used, a two-step loading protocol is employed in which small-molecule agents, which can be irradiated, are loaded first. Following the sterilization step, the biological agents (which FDA has determined should not undergo radiation) are loaded aseptically. The following protocol was developed:
-
1.
Reservoirs are loaded with all small-molecule agents.
-
2.
Loaded microdevices are placed into a sterilization pouch with a desiccant pouch and sent to an outside contractor for gamma irradiation.
-
3.
Irradiated devices are loaded in the research pharmacy with the remaining large-molecule biologics under aseptic conditions.
-
4.
Loaded microdevices are placed coaxially inside a hollow inner stylet. The outer needle is placed over the inner stylet.
-
5.
Each needle assembly is placed into a sterilization pouch with a desiccant pouch.
Sterilization & testing
Pouches containing the needle assemblies are then sent to an outside contractor for gamma irradiation (Steris Laboratories). If biological agents are used in the devices, they are not loaded until after this step to prevent destabilization. After gamma irradiation (and biologic loading, if applicable), devices are sent for endotoxin and sterility testing.
In addition to the endotoxin test, devices must also demonstrate zero growth on a sterility test after gamma irradiation [1]. Samples are put into a sterile vessel with various media where they incubate for 14 days and are observed daily for any bacteria growth. If any design or dimensional changes are made, Bacteriostasis & Fungistasis (B&F) testing is also conducted on 10% of devices. This eliminates the possibility of the device itself inhibiting the growth of bacteria or fungi [7]. Once B&F is performed on a version of device, it is no longer required if there are no design, material, or manufacturing changes [4].
For endotoxin and sterility testing, as well as validation, 10% of samples are randomly selected. Testing is conducted using the Chromogenic method, which adds a reagent to the lysate and activates a clotting cascade upon contact with endotoxins [6].
Biologic agent stability testing
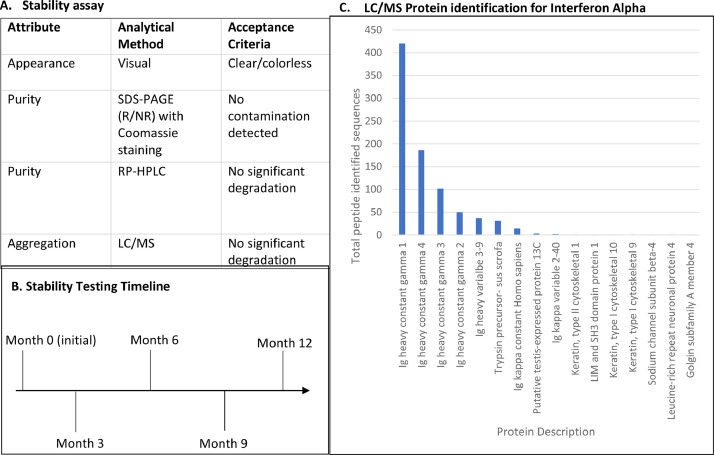
Because biologic agents are typically administered to patients directly from their approved packaging, we encountered the challenge of demonstrating that storage of the biologics in our IMDs would not impact their structural integrity and efficacy. To test our hypothesis that long-term storage of lyophilized biologics in the IMDs would not affect the stability of the drugs, the stability of the biologic agents is assessed every 90 days using a series of tests that were identified in discussions with FDA to indicate that key aspects of biologic compound integrity are maintained Fig. 2. Five such criteria for integrity were identified (Table 1), and for each criteria an industry-standard measurement method was chosen. In these assays the biologics are stored in microdevices and their integrity is compared to the initial compound provided at the initial release date. Stability results were obtained from post-lyophilized samples to provide an accurate baseline for future tests. Table 1 outlines the testing procedures conducted to assess stability of the biological agents used in our clinical studies.
Fig. 2.
Biologic stability assay. (a) Stability of the biologic agents is measured by assessing the appearance, purity, and aggregation. Significant degradation is assessed by collaborators at MIT and Harvard University. (b) Tests are performed at the initial timepoint, and then once every 3 months. (c) Protein identification for Nivolumab at the 3-month timepoint showed endured integrity of the constant and variable regions, with little contamination from outside sources (i.e. trypsin and keratin).
Table 1.
Biological agents’ stability assay.
| Attribute | Analytical Method | Acceptance Criteria |
|---|---|---|
| Appearance | Visual | Clear/colorless |
| Purity | SDS-PAGE (R/NR) with Coomassie staining | No contamination detected |
| Purity | RP-HPLC | No significant degradation |
| Aggregation | LC/MS | No significant degradation |
Fully loaded and sterilized microdevices are stored for up to 3 months at 2-8 °C prior to administration. After 3 months following initial release, the testing procedures described above are performed. If release criteria are met, the batch of devices is released for an additional three-month period. Stability data is provided to the FDA for review prior to using microdevices stored longer than 3 months. Otherwise, the existing microdevice assemblies are discarded and a new batch is made. This is repeated at the 6, 9, and 12-month timepoints.
Devices are stored at the DFCI Research Pharmacy at 2-8 °C for a maximum of one year. Pharmacy personnel keep a detailed record of any fluctuations in external conditions.
Declaration of Competing Interest
O.J. is a consultant to Kibur Medical. His interest was reviewed and is managed by BWH and MGB Healthcare in accordance with their outside interest policies.
Acknowledgments
The authors would like to thank Caroline Harvey and Anita Chung-Thomas of the Dana-Farber Research Pharmacy for their help in the development of IMD protocols.
Funding information
This article is funded by: R01CA232174 (NIH) R21CA216796 (NIH) R37CA224144 (NIH) Director’s Transformative Award (Brigham Research Institute).
Contributor Information
Christine Dominas, Email: cdominas@bwh.harvard.edu.
Oliver Jonas, Email: ojonas@bwh.harvard.edu.
References
- 1.Center for Biologics Evaluation and Research. (2008). Guidance for FDA Reviewers and Sponsors- Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/content-and-review-chemistry-manufacturing-and-control-cmc-information-human-somatic-cell-therapy.
- 2.Food and Drug Administration. (2011). Transfusion and infusion assemblies and similar medical devices. https://www.fda.gov/media/83477/download.
- 3.Franco E., Garcia-Recio V., Jiménez P., Garrosa M., Girbés T., Cordoba-Diaz M., Cordoba-Diaz D. Endotoxins from a pharmacopoeial point of view. Toxins. 2018;10(8) doi: 10.3390/toxins10080331. MDPI AG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Organization for Standardization. (2006, April). Sterilization of health care products — Radiation — Part 1: Requirements for development, validation and routine control of a sterilization process for medical devices. ISO 11137-1:2006. https://www.iso.org/standard/33952.html.
- 5.Jonas O., Landry H.M., Fuller J.E., Santini J.T., Baselga J., Tepper R.I., Cima M.J., Langer R. An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Sci. Transl. Med. 2015;7(284) doi: 10.1126/scitranslmed.3010564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsay G.K., Roslansky P.F., Novitsky T.J. Single-step, chromogenic Limulus amebocyte lysate assay for endotoxin. J. Clin. Microbiol. 1989;27(5) doi: 10.1128/jcm.27.5.947-951.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. (2012, March). 3.2 Test for sterility. https://www.who.int/medicines/publications/pharmacopoeia/TestForSterility-RevGenMethod_QAS11-413FINALMarch2012.pdf.