Key Words: brain-derived neurotrophic factor, cognitive function, dendritic structure, diabetes, hippocampus, insulin resistance, Sirtuin 1, target of rapamycin complex 1
Abstract
In the peripheral nervous system, the activation of Sirtuin 1 can improve insulin resistance; however, the role played by Sirtuin 1 in the central nervous system remains unknown. In this study, rat models of diabetes mellitus were generated by a single injection of streptozotocin. At 8 weeks after streptozotocin injection, the Morris water maze test and western blot assays confirmed that the diabetic model rats had learning and memory deficits, insulin resistance, and Sirtuin 1 expression could be detected in the hippocampus. Insulin and the insulin receptor inhibitor S961 were intranasally administered to investigate the regulatory effects of insulin signaling on Sirtuin 1. The results showed that insulin administration improved the impaired cognitive function of diabetic model rats and increased the expression levels of phosphorylated insulin receptor, phosphorylated insulin receptor substrate 1, and Sirtuin 1 in the hippocampus. Conversely, S961 administration resulted in more severe cognitive dysfunction and reduced the expression levels of phosphorylated insulin receptor, phosphorylated insulin receptor substrate 1, and Sirtuin 1. The Sirtuin 1 activator SRT2104 and the inhibitor Sirtinol were injected into the lateral ventricle, which revealed that the activation of Sirtuin 1 increased the expression levels of target of rapamycin complex 1, phosphorylated cAMP-response element-binding protein, and brain-derived neurotrophic factor. Hippocampal dendritic length and spine density also increased in response to Sirtuin 1 activation. In contrast, Sirtinol decreased the expression levels of target of rapamycin complex 1, phosphorylated cAMP-response element-binding protein, and brain-derived neurotrophic factor and damaged the dendritic structure. These findings suggest that the Sirtuin 1 signaling pathway plays an important role in the development of insulin resistance-related cognitive deficits in diabetic rats. This study was approved by the Animal Ethics Welfare Committee of the First Affiliated Hospital of Hunan University of Chinese Medicine (approval No. ZYFY201811207) in November 2018.
Chinese Library Classification No. R453; R742; R587.2
Introduction
Diabetes is a serious, chronic disease that can lead to complications throughout the body, including the peripheral tissues and central nervous system. Type 1 and type 2 diabetes mellitus can be clinically differentiated based on the lack of insulin production and insulin resistance, respectively. The discovery of central insulin resistance and its influence on cognitive behavior has resulted in the definition of another type of diabetes, referred to as type 3 diabetes (Leszek et al., 2017; Candasamy et al., 2020). Peripheral insulin resistance may trigger insulin resistance in the brain, contributing to neurodegenerative diseases, such as Alzheimer's disease (Rorbach-Dolata and Piwowar, 2019). Diverse pathways, including excitotoxicity, apoptosis, amyloid-beta accumulation, and tau phosphorylation, have been shown to be involved in the development of type 3 diabetes. However, the underlying pathological mechanism of type 3 diabetes remains unclear. Insulin in the brain is responsible not only for energy metabolism (Kleinridders et al., 2014) but also for the maintenance of synaptic plasticity and differentiation (Chiu et al., 2008). Therefore, insulin signaling in the brain may play an important role in the pathophysiology of diabetes-related cognitive declines (Hamer et al., 2019). However, the molecular mechanism underlying brain insulin resistance associated with cognitive dysfunction remains to be fully determined.
Sirtuin 1 (SIRT1) is a deacetylase protein that has been found to be expressed in the liver, skeletal muscle, pancreas, adipose tissues, and brain. A wealth of data has suggested that SIRT1 might play an important role in insulin resistance and type 2 diabetes (Cao et al., 2016; Zhang, 2020). For example, activated SIRT1 may prevent high-fat diet-induced hepatic triglyceride accumulation and oxidative liver damage, which have been implicated in the pathophysiology of insulin resistance and type 2 diabetes (Valdecantos et al., 2012). In addition, the activation of SIRT1 in β-cells may increase glucose sensing and insulin secretion (Luu et al., 2013). However, whether the central insulin resistance-mediated cognitive dysfunction associated with diabetes involves SIRT1 remains unclear.
A recent study suggested that SIRT1 might play important roles in diabetes-induced cognitive impairment through the regulation of diverse cellular processes, including deacetylation, neurite outgrowth, and mitochondrial function (Cao et al., 2017). Changes in dendritic spines can have significant effects on the connection patterns between neuronal circuits and cognitive behavior. Lee et al. (2011) demonstrated that insulin signaling promotes hippocampal synaptic plasticity through the activation of the downstream signaling pathways. Liu et al. (2013) found that insulin promotes neurite outgrowth by regulating SIRT1 expression in SH-SY5Y cells. However, the mechanism underlying SIRT1 signaling pathway-associated damage to dendritic structures in diabetic model rats with cognitive deficits remains to be fully determined.
In this study, we investigated the role played by SIRT1 in brain insulin resistance-mediated, diabetes-induced cognitive dysfunction. We first tested the learning and memory functions of diabetic model rats and analyzed the expression patterns of SIRT1 and proteins associated with insulin signaling. Then, we examined the effects of activating or inhibiting hippocampal insulin signaling on cognitive impairments and SIRT1 expression. Finally, we explored the molecular mechanisms through which the SIRT1 signaling pathway affects the hippocampal dendritic spines of diabetic rats with cognitive impairments.
Materials and Methods
Animals
A total of 118 male Sprague Dawley rats (six weeks old, weighing 180–220 g) were purchased from Hunan Slack Scene of Laboratory Animal Company (Changsha, China; certificate No. SCXK (Xiang) 2016-0002) and maintained in a specific pathogen-free Laboratory Animal Center at the First Affiliated Hospital of Hunan University of Chinese Medicine. All rats were maintained on a 12-hour light/dark cycle in an air-conditioned room with constant temperature (23 ± 1°C) and free access to food and water. All procedures performed on animals were approved by the Animal Ethics Welfare Committee of the First Affiliated Hospital of the Hunan University of Chinese Medicine (approval No. ZYFY201811207) in November 2018. All experiments were designed and reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Experimental design
The Sprague-Dawley rats were randomized into diabetes and control groups. Type 1 diabetic model rats were generated by performing a single tail vein injection of streptozotocin (STZ; Solarbio, Beijing, China), as described in a previous study (Kamal et al., 1999). Briefly, STZ (40 mg/kg) was dissolved in citric acid buffer (pH 4.9), chilled to 4°C, and then slowly injected into the rats (n = 20). The control group rats (n = 15) were injected with the citric acid buffer (2 mL/kg). Three days after the STZ injection, blood glucose levels were determined using tail tip blood. Rats with blood glucose levels > 16 mM were considered to be successfully modeled diabetic rats (Yang et al., 2018). Fifteen diabetic rats were fed a normal diet for 8 weeks, and their blood glucose levels were measured using a blood glucose sensor (Sinocare, Changsha, China) at weeks 2, 4, 6, and 8. At 8 weeks after the rats developed diabetes, the cognitive functions of the diabetic model rats were measured using the Morris water maze (MWM) test (Figure 1). The hippocampal dendritic structures were examined by Golgi staining, and insulin signaling-related proteins and SIRT1 protein expression were detected by western blot assay.
Figure 1.
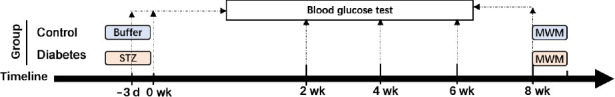
The first flow chart.
MWM: Morris water maze test; STZ: streptozotocin.
Then the effects of hippocampal insulin signaling on cognitive behaviors and the expression of SIRT1 protein were investigated in diabetic model rats. Sixty-six STZ-induced diabetic model rats were fed a normal diet for 8 weeks, and six normal rats received a citric acid buffer injection and were fed a normal diet. The expression of insulin signaling-related proteins and SIRT1 protein were detected by western blot assay in the hippocampus of 6 diabetic model rats and all normal rats. Intranasal insulin (1 U/25 μL; Novo Nordisk, Copenhagen, Denmark) was administered to 15 diabetic model rats once daily for 6 weeks. The same number of diabetic rats received intranasal saline (20 μL) as a vehicle treatment. S961 (1 μg/20 μL; MedChemExpress, Monmouth Junction, NJ, USA), an insulin receptor inhibitor, was administered to another 15 diabetic model rats once daily for 7 days, and the same number of diabetic model rats received intranasal saline (20 μL). For intranasal delivery, each rat was held in the manner of “over the shoulder grip” with one hand, stabilized against the handler (Machholz et al., 2012). The material was placed at the nares of the rat using a syringe. The rat was restrained until the material disappeared into the nares. After administration, the cognitive behaviors of the rats were measured, and related protein measurements were performed (Figure 2).
Figure 2.
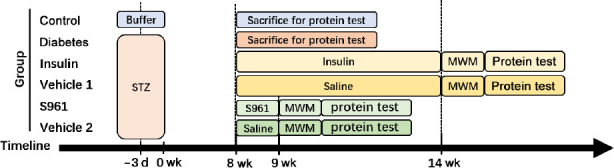
The second flow chart.
MWM: Morris water maze test; STZ: streptozotocin.
Finally, the involvement of SIRT1 in the regulation of hippocampal structure was assessed, and the downstream molecules of SIRT1 were investigated. Twenty-eight STZ-induced diabetic model rats and four normal rats were used for this experiment. After feeding for 8 weeks, the hippocampal dendritic structures of four diabetic model rats and all normal rats were examined by Golgi staining. SRT2104 (10 μM, MedChemExpress), a SIRT1 activator, was intracerebroventricularly injected into six diabetic model rats for 16 days. One rat died during this experiment (Abe-Higuchi et al., 2016). The vehicle-treated diabetic model rats received phosphate-buffered saline (10 μL) for the same time. Sirtinol (10 μM, MedChemExpress), a SIRT1 inhibitor, was intracerebroventricularly injected into six diabetic model rats for 14 days, and one rat died (Abe-Higuchi et al., 2016). The vehicle-treated diabetic model rats received phosphate-buffered saline (10 μL) for the same time, and one rat died. After administration, the hippocampal dendritic structures were detected by Golgi staining, and potential downstream molecules were tested by western blot assay (Figure 3).
Figure 3.
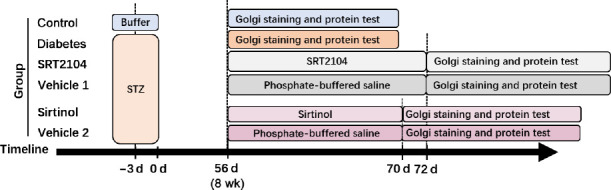
The third flow chart.
STZ: Streptozotocin.
Morris water maze test
The MWM test was used to analyze the learning and memory functions of rats (Morris, 1981). The test apparatus (Panlab Smart 3.0; Panlab, Barcelona, Spain) was a circular black tank (200 cm in diameter) filled with clear water (25 ± 1°C). The pool was then divided into four equal quadrants, labeled A, B, C, and D. A clear plexiglass platform was used and submerged into the water such that it was invisible from the surface of the water (Bromley-Brits et al., 2011). The platform was placed in quadrant A for the first 4 days and was removed on the fifth day. During the hidden platform test, the time required for rats to climb onto the submerged platform was recorded as the escape latency to assess the learning abilities of the rats. The rats were placed on the platform and allowed to remain there for 10 seconds if they failed to reach the platform within 60 seconds. The platform was removed on the 5th day for the probe test. The time spent in the target quadrant, the number of times crossing the previous platform location, the average speed, and the percentage of total swimming distance in the target quadrant were recorded to assess the memory functions of rats.
Golgi staining
Golgi staining was performed to investigate neuronal morphology (Abe-Higuchi et al., 2016). After the behavior tests, the rats were anesthetized by pentobarbital sodium (Merck, Darmstadt, Germany) and euthanized. The brains were cut into approximately 3 mm-thick coronal original sections using a scalpel, and then the original sections containing hippocampus tissue were immersed in Golgi solution (Servicebio, Beijing, China). After immersion for 48 hours, the original solution was replaced with fresh solution. The solution was refreshed every 3 days for 14 days of immersion, and then the brain tissue was placed into 15% and 30% sucrose solution and incubated at 4°C for dehydration for 1 day and 2 days, respectively. The tissue was then transferred to concentrated ammonia for 45 minutes. After washing for 1 minute, the tissue was treated with an acid-hardening fixative for 45 minutes. After washing for 1 minute, the tissue was dehydrated in 30% sucrose solution at 4°C protected from light for 2 days. The tissue was embedded in OCT (Sakura, Torrance, CA, USA) and placed in a freezing microtome (Thermo, Waltham, MA, USA) for freezing, and microtome blades (Leica, Wetzlar, Germany) were used to cut 100-μm-thick brain sections, which were placed on gelatin slides. Each section was stored in a slide box at room temperature in the dark overnight. The slices were immersed in pure water for 20 seconds. After blotting excess water from around the tissues with filter paper, the slides were sealed with glycerin gelatin. Finally, the dendritic structures were imaged using a Nikon Eclipse E100 microscope (Nikon, Tokyo, Japan) and analyzed using ImageJ 1.52p software (National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
Western blot analysis was performed as described in our previous study (Yang et al., 2018). Briefly, proteins from the hippocampus were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes (Millipore Corporation, Billerica, MA, USA), and blocked in Tris-buffered saline containing 0.05% Tween-20 and 1% non-fat dry milk for 60 minutes. Polyvinylidene fluoride membranes were incubated primary antibodies at 4°C overnight, including anti-insulin receptor (IR; rabbit anti-rat; 1:1000; Cat# AF6099; Affinity Biosciences, Cincinnati, OH, USA), anti-p-IR (rabbit anti-rat; 1:1000; Cat# AF3099; Affinity Biosciences), anti-p-IR substrate-1 (IRS-1; rabbit anti-rat; 1:1000; Cat# AF4424; Affinity Biosciences), anti-IRS-1 (rabbit anti-rat; 1:1000; Cat# 2382; Cell Signaling, Danvers, MA, USA), anti-SIRT1 (rabbit anti-rat; 1:1000; Cat# BF0189; Affinity Biosciences), anti-target of rapamycin complex 1 (TORC1; rabbit anti-rat; 1:1000; Cat# 10441-1-AP; Proteintech, Chicago, IL, USA), anti-p-cAMP-response element-binding protein (p-CREB; rabbit anti-rat; 1:1000; Cat# 9197; Cell Signaling), anti- CREB (rabbit anti-rat; 1:1000; Cat# 9198; Cell Signaling), anti-brain-derived neurotrophic factor (BDNF; rabbit anti-rat; 1:2000; Cat# 47808; Cell Signaling), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (rabbit anti-rat; 1:2000; Cat# 7074; Cell Signaling). The membranes were washed with Tris-buffered saline plus 0.05% Tween-20 and incubated with horseradish peroxidase secondary antibody (goat anti-rabbit; 1:3000; Cat# 7074; Cell Signaling). Finally, the polyvinylidene fluoride membranes were developed using Enhanced Chemiluminescence Reagents (New Cell & Molecular Biotech, Nanjing, China), according to the manufacturer's instructions. The optical densities of the bands were calculated by Image Lab (Bio-Rad, Hercules, CA, USA).
Statistical analysis
The effects of diabetes duration on blood glucose levels were analyzed by one-way repeated-measures analysis of variance. The learning efficacy during the MWM was analyzed using a general linear model (GLM). The other indicators were all analyzed by one-way analysis of variance. The least significant difference (LSD) test was used when the variances were homogenous (P < 0.05), and the Dunnett test was used when the variances were not homogenous (P < 0.05). Statistical analysis of all data was performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Differences were considered significant at a P-value of < 0.05.
Results
Cognitive dysfunction in STZ-induced diabetic model rats
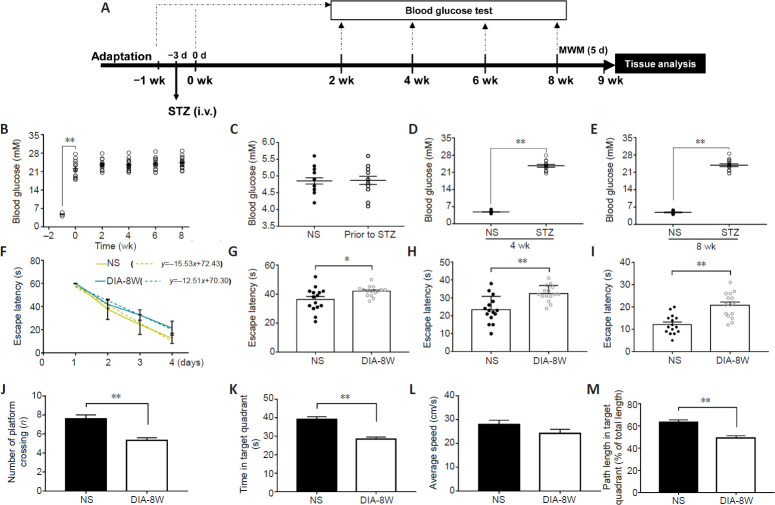
STZ is a commonly used drug to induce diabetes in animal models (Lenzen, 2008). We used the method of a single intravenous tail vein STZ injection of STZ to establish a rat model of diabetes and measured the cognitive functions after the diabetic model rats were fed a normal diet for 8 weeks. First, we monitored the effects of diabetes duration on blood glucose levels (Figure 4A). As shown in Figure 4B, after 3 days of the STZ injection, the blood glucose levels of rats increased significantly compared with before STZ injection (P < 0.01). However, no significant differences in blood glucose levels were observed 2, 4, 6, and 8 weeks after the rats developed diabetes. In addition, we compared the blood glucose levels between diabetic model rats and age-matched control rats prior to STZ injection and 4 and 8 weeks after the rats developed diabetes. Before the STZ injection, no difference was observed in the blood glucose levels between diabetic model rats and control rats (Figure 4C). At weeks 4 and 8, the blood glucose levels of diabetic model rats were significantly higher than those in the age-matched control rats (both P < 0.01; Figure 4D and E).
Figure 4.
Cognitive deficits in streptozotocin (STZ)-induced diabetic model rats at week 8 of diabetes duration.
(A) Schematic diagram of the experimental schedule. Diabetes was induced by the intravenous (i.v.) injection of STZ. Blood glucose levels of diabetic rats (n = 15) were measured prior to diabetes induction, 3 days after STZ injection, and 2, 4, 6, and 8 weeks after the rats developed diabetes. Blood glucose was tested at weeks 4 and 8 in age-matched non-STZ-treated rats (NS) (n = 15). Learning and memory functions were tested by the Morris water maze (MWM) after 8 weeks of diabetic modeling. (B) The effects of diabetes duration on blood glucose levels in diabetic model rats (n = 15). (C–E) Blood glucose of diabetic or NS rats before STZ injection (C) and at weeks 4 (D) and 8 (E) of diabetes modeling. The effects of diabetes duration on blood glucose levels were analyzed by one-way repeated-measures analysis of variance, whereas the between-group differences in blood glucose levels were analyzed by one-way analysis of variance. (F–M) The cognitive functions of diabetic rats (DIA-8W) or NS rats at week 8 of diabetes modeling were measured by MWM (n = 15 per group). The learning function was evaluated using the hidden platform test. The escape latency time during the first 4 days was used to generate the learning curve (F), and detailed data for days 2, 3, and 4 are shown (G–I). The memory functions of rats were measured in the probe test. The number of times that the rats crossed the expected platform location (J), the time spent in the target quadrant (K), the average swimming speed of rats (L), and the percentage of swimming distance in the target quadrant (M) were recorded. In the MWM experiments, the learning efficiency was analyzed by general linear model, and the differences in daily learning times were analyzed by one-way analysis of variance during the hidden platform test. The time spent in the target quadrant, the number of times that the rats crossed the expected platform location, the average swimming speed of rats, and the percentage of swimming distance in the target quadrant during the probe test were analyzed by one-way analysis of variance. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01. The experiments were repeated three times.
We used the MWM test to evaluate the learning and memory functions of diabetic model rats 8 weeks after STZ injection. First, in the hidden platform test, the learning abilities of the diabetic model rats were evaluated. As shown in Figure 4F, we used the slope of the learning curve to evaluate the rats learning functions. The learning function of diabetic model rats was significantly worse than that of age-matched control rats (P < 0.01), with diabetic model rats spending longer times to find the underwater platform on days 2 (P < 0.05), 3 (P < 0.01), and 4 (P < 0.01; Figure 4G–I), indicating a significant decrease in learning function after 8 weeks of diabetes modeling.
We used the probe test to evaluate the spatial memory abilities of diabetic model rats, which spent significantly decreased time in the target quadrant (P < 0.01; Figure 4J), crossed the expected platform location fewer times (P < 0.01; Figure 4K), and had a lower percentage of total swimming distance in the target quadrant (P < 0.01; Figure 4M) compared with rats in the control group. However, compared with the control group, diabetic model rats did not show any significant decrease in the average swimming speed (P > 0.05; Figure 4L). These results revealed disruptions in learning and memory formation in rats after 8 weeks of diabetic modeling.
Brain insulin resistance and reduced SIRT1 expression contribute to cognitive dysfunction in diabetic model rats
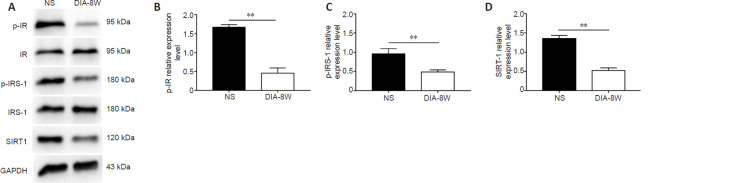
In the brain, insulin exerts biological effects by acting on the IR and IRS, which are components of the insulin signaling pathway. Brain insulin signaling has been suggested to play an important role in cognitive function (Sukhov et al., 2020). Therefore, we investigated the phosphorylation of the IR and IRS-1 in the hippocampus of diabetic model rats with cognitive dysfunction (Figure 5A). We found that the expression levels of p-IR and p-IRS-1 in the hippocampus of diabetic model rats were reduced at week 8 (P < 0.01, vs. control group; Figure 5B and C). In addition, the expression levels of SIRT1, which is related to insulin resistance in the periphery, were also decreased in the hippocampus (P < 0.01; Figure 5D). These results suggested the potential contribution of brain insulin resistance and reduced SIRT1 expression to the observed cognitive deficits in diabetic model rats.
Figure 5.
Insulin signaling molecules and SIRT1 expression are reduced in diabetic rats at week 8 of diabetes.
(A) Bands of p-IR, p-IRS-1, and SIRT1. (B–D) Relative expression levels of p-IR (B), p-IRS-1 (C), and SIRT1 (D). The relative expression levels are expressed as the optical density ratio compared against GAPDH levels. Data are presented as the mean ± SEM (n = 6 per group). **P < 0.01 (one-way analysis of variance). The experiments were repeated three times. DIA-8W: Diabetic rats at week 8 of diabetes; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; IR: insulin receptor; IRS-1: IR receptor substrate 1; NS: non-streptozotocin rats; p-IR: phospho-insulin receptor; p-IRS-1: phospho-insulin receptor substrate; SIRT1: Sirtuin 1.
Insulin administration improves cognitive function, activates insulin signaling, and increases the SIRT1 protein level in diabetic model rats
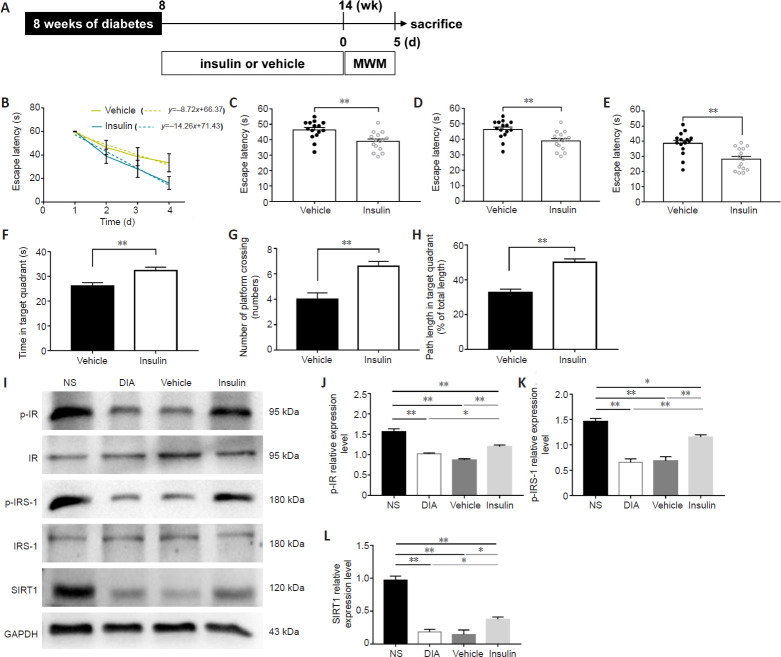
To confirm whether brain insulin resistance is involved in the cognitive functions of diabetic model rats, STZ-induced diabetic model rats were maintained for 8 weeks and then intranasally administered insulin for 6 weeks (Figure 6A), after which the MWM test was performed. We found significantly improved learning and memory functions in diabetic model rats treated with insulin compared with normal saline-managed diabetic model rats. In the hidden platform test, insulin treatment significantly improved rat learning impairments (P < 0.01; Figure 6B) and decreased the time required to find the platform on days 2, 3, and 4 in diabetic model rats (P < 0.01; Figure 6C–E). In the probe test, we found a significantly longer swimming time and path length in the target quadrant, and an increase in the number of times crossing the expected platform location in rats that received insulin compared with rats treated with vehicle (P < 0.01; Figure 6F–H). Our data indicated that insulin administration improved the cognitive functions of diabetic rats.
Figure 6.
Intranasal insulin administration alters cognitive dysfunction and activates insulin signaling.
(A) Schematic diagram of the experimental schedule. After 8 weeks of diabetes, diabetic rats were treated with insulin or vehicle for 6 weeks, and rat behaviors were investigated by the MWM (n = 15 per group). (B–E) Learning functions were evaluated by the escape latency time over 4 days (B). The latency time on days 2 (C), 3 (D), and 4 (E) were analyzed. (F–H) The memory functions of rats were assessed by the time spent in the target quadrant (F), the number of times that the rats crossed the expected platform location (G), and the percentage of distance in the target quadrant (H). (I–L) Protein expression levels were examined (n = 6 per group). Insulin administration increased the expression of p-IR (optical density ratio to IR) (J), p-IRS-1 (optical density ratio to IRS-1) (K), and SIRT1 (optical density ratio to GAPDH) (L) in the hippocampus of diabetic rats with cognitive decline. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01 (one-way analysis of variance). The experiments were repeated 3 times. DIA: Diabetic rats; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; IR: insulin receptor; IRS-1: IR receptor substrate 1; MWM: Morris water maze test; NS: non-streptozotocin rats; p-IR: phospho-insulin receptor; p-IRS-1: phospho-insulin receptor substrate; SIRT1: Sirtuin 1.
Using a western blot assay, we found that diabetic model rats treated with insulin showed the increased expression levels of p-IR (P < 0.01; Figure 6I and J), p-IRS1 (P < 0.01; Figure 6I and K), and SIRT1 (P < 0.05; Figure 6I and L) compared with those in the vehicle-treated rats. These results suggested that the activation of insulin signaling in the hippocampus increases the expression levels of SIRT1 protein. No significant differences in protein expression levels were observed between untreated diabetic model rats and vehicle-treated rats, indicating that the intranasal administration method had no specific effects on the examined proteins.
Pharmacologic inhibition of insulin receptor aggravates cognitive dysfunction and decreases SIRT1 protein levels in diabetic model rats
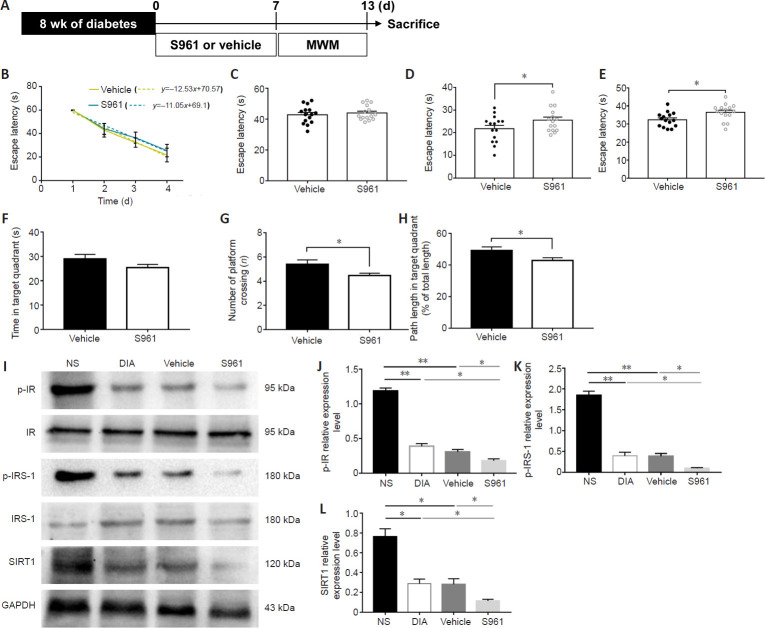
We next assessed the effects of S961, an insulin signaling inhibitor (Sharma and Kumar, 2018), on the cognitive function of diabetic rats (Figure 7). STZ-induced diabetic model rats were maintained for 8 weeks and then intranasally administered insulin for 7days (Figure 7A). As shown in Figure 7B, diabetic model rats treated with S961 had similar learning curves as vehicle-treated diabetic model rats (P > 0.05). However, on days 3 and 4 of the hidden platform test, diabetic model rats treated with S961 showed significantly longer times finding the platform (P < 0.05; Figure 7D and E). In the probe test, S961 treatment significantly decreased the number of times crossing the expected platform location and significantly reduced the swimming distance in the target quadrant (P < 0.05; Figure 7G and H).
Figure 7.
Insulin receptor inhibitor S961 aggravates the impairment of cognitive function in diabetic model rats and inhibits insulin signaling and SIRT1 expression.
(A) Schematic diagram of the experimental schedule. After 8 weeks of diabetes, diabetic rats were administered S961 or vehicle for 7 days, and rat behaviors were investigated by MWM (n = 15 per group). (B–H) Behavioral data. (B) The learning curve. (C–E) The escape latency time on days 2 (C), 3 (D), and 4 (E). (F) The time spent in the target quadrant, (G) the number of times crossing the expected platform location, and (H) is the percentage of swimming distance in the target quadrant. (I–L) S961 decreased the expression of p-IR (optical density ratio to IR) (J), p-IRS-1 (optical density ratio to IRS-1) (K), and SIRT1 (optical density ratio to GAPDH) (L) in the hippocampus of diabetic model rats with cognitive decline (n = 6 per group). Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01 (one-way analysis of variance). The experiments were repeated three times. DIA: Diabetic rats; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; IR: insulin receptor; IRS-1: insulin receptor substrate-1; MWM: Morris water maze test; NS: non-streptozotocin rats; p-IR: phospho-insulin receptor; p-IRS-1: phospho-insulin receptor substrate-1; SIRT1: Sirtuin 1.
We then measured the protein levels of p-IR, p-IRS1, and SIRT1 (Figure 7I). Western blot analysis showed that the expression levels of p-IR (P < 0.05; Figure 7J), p-IRS1 (P < 0.05; Figure 7K), and SIRT1 (P < 0.05; Figure 7L) were significantly reduced in the hippocampus of diabetic model rats treated with S961 compared with the protein levels in vehicle-treated diabetic model rats. These results suggest that the inhibition of insulin signaling in the hippocampus aggravated memory dysfunction and decreased SIRT1 expression levels.
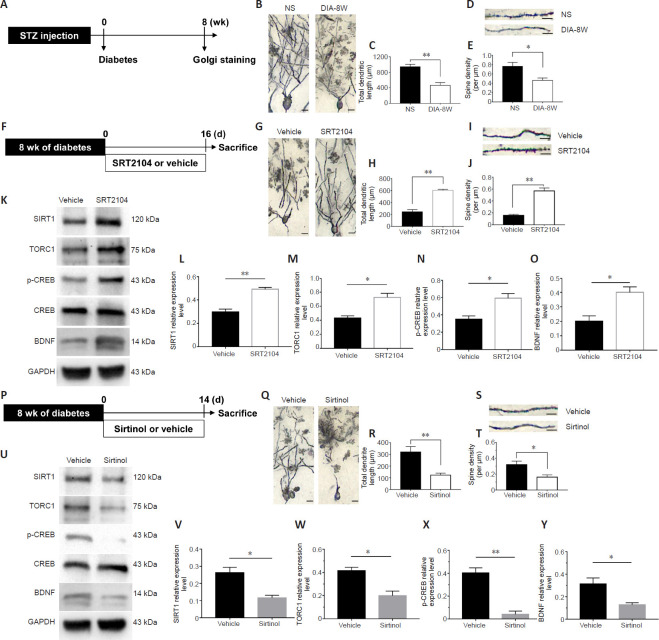
SIRT1/TORC1 signaling regulates hippocampal structural plasticity in diabetic model rats with cognitive deficits
Although an earlier study demonstrated that brain insulin resistance elicited by diabetes alters the synaptic and dendritic structure (Choi et al., 2005), the downstream target of insulin signaling remains unknown. Therefore, we investigated whether SIRT1 activation could block diabetes-related changes to dendritic structures. First, the dendritic structures of the hippocampus in diabetic rats were confirmed by performing Golgi staining in STZ-induced diabetic model rats that were maintained for 8 weeks. This staining revealed reduced dendritic lengths and spine densities in the hippocampus of diabetic model rats with cognitive decline (P < 0.01 and P < 0.05; Figure 8A–E). Next, the involvement of SIRT1 in the regulation of the hippocampal structure was assessed. STZ-induced diabetic model rats were maintained for 8 weeks, and then the rats were injected with SRT2104, a SIRT1 activator, for 16 days. We found that SRT2104 significantly increased the dendritic lengths and spine densities in the hippocampus of diabetic model rats (both P < 0.01; Figure 8F–J). Conversely, the administration of Sirtinol, a SIRT1 inhibitor, for 14 successive days reduced the hippocampal dendritic lengths and increased spine loss (P < 0.01 or P < 0.05; Figure 8P–T).
Figure 8.
SIRT1 signaling pathway is associated with the dendritic structure in the hippocampus of diabetic rats.
(A) Schematic diagram of the experimental schedule. After 8 weeks of diabetes modeling, the hippocampal dendritic structures of diabetic model rats (DIA-8W) or non-STZ-treated rats (NS) were assessed by Golgi staining. (B) Representative images of hippocampal neurons in DIA-8W rats or NS rats. The dendritic lengths were reduced in the hippocampus of diabetic rats compared with non-STZ-treated rats. (C) The total dendrite lengths of hippocampal neurons (n = 4 per group). (D) Representative images of dendritic spines in hippocampal neurons from DIA-8W rats or NS rats. The spine densities were reduced in the hippocampus of diabetic model rats compared with non-STZ-treated rats. (E) Spine densities (n = 4 per group). (F) Schematic diagram of the experimental schedule. (G–O) Diabetic model rats were treated with SRT2104 or vehicle for 16 days, and then the hippocampal dendritic structures were examined by Golgi staining (G–J), and the proteins were analyzed by western blot assay (K–O). (G) Representative images of hippocampal neurons in rats injected with SRT2104 or vehicle. Compared with vehicle-treated rats, SRT2104 treatment significantly increased the dendritic lengths in the hippocampus of diabetic model rats. (H) The total dendrite length of neurons (n = 4 per group). (I) Representative images of dendritic spines in rats injected with SRT2104 or vehicle. Compared with vehicle-treated rats, SRT2104 significantly increased the spine densities in the hippocampus of diabetic model rats. (J) Spine densities (n = 4 per group). (K–O) SRT2104 increased the expression of SIRT1 (L), TORC1 (M), p-CREB (N), and BDNF (O) in the hippocampus of diabetic rats with cognitive dysfunction (n = 5–6 per group). (P) Schematic diagram of the experimental schedule. Diabetic rats were treated with SRT2104 or vehicle for 16 days. (Q–Y) The hippocampal dendritic structure was tested by Golgi staining (Q–T), and the proteins were analyzed by western blot assay (U–Y). (Q) Representative images of hippocampal neurons in rats injected with Sirtinol or vehicle. Sirtinol treatment reduced the dendritic lengths in the hippocampus of diabetic model rats. (R) The total dendrite length of neurons (n = 4–6 per group). (S) Representative images of dendritic spines in rats injected with Sirtinol or vehicle. Sirtinol decreased the spine loss in the hippocampus of diabetic model rats. Scale bars: 50 μm in B, G, and Q; 20 μm in D, I, and S. (T) Spine densities (n = 4–6 per group). (U–Y) Sirtinol decreased the expression of SIRT1 (V), TORC1 (W), p-CREB (X), and BDNF (Y) in the hippocampus of diabetic model rats with cognitive dysfunction (n = 4–6 per group). p-CREB relative expression was expressed as the optical density ratio to CREB, and the relative expression levels of other proteins were expressed as the optical density ratio to GAPDH. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01 (one-way analysis of variance). The experiments were repeated 3 times. BDNF: Brain-derived neurotrophic factor; CREB: cAMP-response element-binding protein; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; p-CREB: phosphorylated cAMP-response element-binding protein; SIRT1: Sirtuin 1; Sirtinol: SIRT1 inhibitor; SRT2104: SIRT1 activator; STZ: streptozotocin; TORC1: target of rapamycin complex 1.
We also investigated the molecular pathways underlying the SIRT1/TORC1 signaling. We found that SRT2104 increased SIRT1, TORC1, p-CREB, and BDNF levels in the hippocampus (P < 0.01 or P < 0.05; Figure 8K–O). Conversely, we also found that the infusion of Sirtinol into the hippocampus of diabetic model rats reduced the levels of these proteins (P < 0.01 or P < 0.05; Figure 8U–Y). These data suggested that SIRT1 signaling may be involved in hippocampal structural plasticity, which is damaged in diabetic model rats.
Discussion
The results of this study provided insights into the mechanistic links between hippocampal insulin signaling and cognitive function in STZ-induced diabetic model rats. The learning and memory capabilities of diabetic model rats were significantly damaged 8 weeks after the STZ injection. Moreover, the decreased expression levels of p-IR, p-IRS-1, and SIRT1 in the hippocampus of diabetic rats were revealed by western blot assay. However, cognitive deficits in diabetic model rats were prevented by insulin administration, whereas more severe cognitive deterioration was induced by S961 administration. Insulin administration activates the insulin signaling pathway, increasing the SIRT1 protein level. Conversely, the inhibition of insulin signaling resulted in the decreased expression of SIRT1 protein. These results suggested that SIRT1 protein may be involved in brain insulin resistance, which mediates diabetes-related cognitive dysfunction. In addition, aberrant dendritic structures were observed in the hippocampus of rats suffering from 8 weeks of diabetes duration. Treatment with a SIRT1 activator protected dendritic structures and increased TORC1, p-CREB, and BDNF protein levels, whereas the inhibition of SIRT1 reduced dendritic lengths and spine densities, which was accompanied by decreased TORC1, p-CREB, and BDNF levels. Thus, our findings suggested that brain insulin resistance drives diabetes-related cognitive decline through the inhibition of SIRT1 signaling.
The effects of diabetes on cognitive function have been reported in many studies (Bhutada et al., 2012; Ye et al., 2018; Tabassum et al., 2020). For instance, male rats fed with a high-fat diet and given a 35 mg/kg STZ injection to induce a type 2 diabetic model showed significant deficits in spatial learning and memory function in the MWM test after 9 weeks (Ye et al., 2018). In another study, a type 1 diabetes mellitus model was induced by a single injection of 60 mg/kg STZ, and these rats exhibited impaired performance in the MWM test and a reduced investigation ratio in the novel object recognition task after 30 days of modeling (Bhutada et al., 2012). The doses of STZ, the method of STZ injection, and the duration of diabetes modeling differ across studies. Kamal et al. (1999) found a progressive deficit in synaptic plasticity, which is associated with functional cognitive deficits and can be observed after a diabetes duration of 6 or 8 weeks. A similar method was used in this study. The blood glucose levels of the diabetic model rats increased throughout the entire experiment performed in our study. Different from our results, studies (Kamal et al., 1999; Luu et al., 2013) reported a slight decline in blood glucose levels from 6 to 8 weeks of diabetic modeling. One potential explanation for this difference is that we replaced saline with citric acid buffer as the solvent for STZ. The pH value is an obvious difference between saline (pH 7) and the citric acid buffer (pH 4.9). As a result, the biological activity of STZ is higher when dissolved in citric acid buffer compared with that in saline. Thus, in our experiment, the pancreatic island cells were more severely damaged, and the phenomenon in which the blood glucose levels of diabetic rats remained high until the eighth week was observed.
Next, the learning and memory functions of diabetic rats were analyzed through two procedures of the MWM test, including the hidden platform test and the probe test. In the hidden platform test, the escape latency for rats to find the platform is used to evaluate the learning function of rats. Currently, these data are commonly analyzed by one-way analysis of variance (Vorhees and Williams, 2006; Bhutada et al., 2012; Li et al., 2017). The one-way analysis of variance is also incorporated into the language of the general linear model (GLM) (Scott et al., 2014). In the hidden platform test, a one-way analysis of variance can only be used to analyze the learning of rats for each day, separately, and is unable to identify changes in the learning ability over consecutive days. In this experiment, GLM analysis, which has not previously been used to analyze the results of the MWM test before, was added to calculate the learning curves of the rats during the training period of the hidden platform test to estimate learning efficiency. The results showed that the time required for diabetic rats learn the location of the hidden platform decreased much more slowly than was observed for normal rats, indicating that diabetic rats displayed a worse learning ability than normal rats. The spatial memory of diabetic model rats was assessed by the probe test. Compared with the control rats, diabetic model rats showed no significant changes in swimming speeds. One possible explanation for this result may be that the swimming speed reflects exercise ability, not memory ability. However, diabetic model rats showed significant differences in swimming times in the target quadrant, the number of times crossing the expected location of the target platform, and the percentage of swimming distance in the target quadrant compared with the age-matched control rats. Thus, diabetic model rats can be used to investigate the mechanism of diabetes-related cognitive impairment after 8 weeks of diabetes modeling.
Insulin resistance is closely related to cognitive impairments, and the activation of brain insulin signaling can effectively improve cognitive decline (Spinelli et al., 2019). Intranasal insulin administration facilitates memory abilities not only in Alzheimer's disease patients but also in healthy individuals (Reger et al., 2008). Similarly, intranasal insulin treatment was found to significantly improve the deteriorated memory functions in intracerebroventricularly STZ-injected rats, which serves as a sporadic Alzheimer's disease animal model (Rajasekar et al., 2017). In this study, after intranasal insulin treatment for 6 consecutive weeks, diabetic model rats showed significantly improved learning and memory functions compared with vehicle-treated rats. Sukhov et al. (2020) also showed that insulin treatment improved the spatial memory of neonatal rats with diabetes mellitus. In contrast, S961 administration induced more severe cognitive dysfunction in diabetic model rats. S961 is a type of biosynthetic IR antagonist that was found to downregulate the phosphorylation of IR in the liver, muscle, kidney, and brain (Ruegsegger et al., 2019; Meijer and Barrett, 2021). IRS-1 is a downstream molecular of IR. In this study, the phosphorylation of IR and IRS-1 were significantly reduced in the hippocampus of diabetic model rats with cognitive impairment. In addition, IR and IRS-1 phosphorylation were increased in diabetic rats that received insulin administration and decreased after S961 treatment. Thus, the dysfunction of brain insulin signaling, also known as IR/IRS-1 signaling, is involved in diabetes-related cognitive decline.
A recent study suggested that SIRT1 might play an important role in the treatment of cognitive decline induced by diabetes (Cao et al., 2017), and several studies have confirmed that SIRT1 plays a central role in metabolic regulation and insulin sensitivity in the liver, muscle and adipose tissue (Shen et al., 2018; Zhang et al., 2018; Shan et al., 2020; Sun et al., 2020). However, the relationship between insulin signaling and SIRT1 remains uncertain and controversial in the central nervous system. In our studies, the intranasal administration of insulin significantly increased SIRT1 expression in the hippocampus of diabetic model rats with cognitive impairment. However, the IR inhibitor significantly reduced the expression of SIRT1. To date, brain insulin signaling has been reported to impact hippocampus-dependent cognitive tasks through a variety of molecular cascades, such as phosphoinositide-3-kinase (PI3K)/protein kinase B (AKT), mitogen-activated protein kinase (MAPK), mammalian target of rapamycin (mTOR), and CREB (Zeng et al., 2016; Spinelli et al., 2019). Niyomchan et al. (Niyomchan et al., 2015) found that insulin promoted neurite outgrowth via the activation of PI3K/AKT/SIRT1 signaling. In addition, animal studies have shown that insulin signaling promotes dendritic arbor development by activating Ras/MAPK signaling (Banks et al., 2012), which could activate SIRT1 to protect endothelial cells from oxidative stress (Marampon et al., 2016). Thus, the results from our study and those from other studies have indicated that hippocampal insulin signaling might positively regulate the expression of SIRT1. However, some studies have shown that activated SIRT1 improves insulin sensitivity (Cao et al., 2017) and increases the expression of proteins related to the insulin signaling pathway (Yoshizaki et al., 2009). Therefore, interactions may occur between insulin signaling and SIRT1 in the brains of diabetic rats. The relationship between insulin signaling and SIRT1 deserves to be further studied in our future work by researching the effects of SIRT1 activation and inhibition on brain insulin signaling.
Abnormal dendritic spine structures are a prominent feature associated with cognitive dysfunction (Torres et al., 2018). Defects in both spine morphology and spine density correlate with cognitive decline in Alzheimer's disease, dementia, and other neurodegenerative disorders (Penzes et al., 2011; Pchitskaya et al., 2018). Our study showed significantly shorter dendritic branches and severe spine loss in diabetic rats with cognitive dysfunction using the Golgi staining method. Fan et al. (2019) also reported changes in dendritic structural plasticity in prediabetic rats associated with cognitive dysfunction, which was consistent with our findings. Brain insulin resistance results in a decrease in the spine density in the hippocampus, which was related to cognitive decline (Lee et al., 2011). However, the molecular mechanisms through which brain insulin signaling regulates the morphology of dendritic spines remains unclear. Research has revealed that insulin promotes dendritic spine formation in hippocampal neurons (Lee et al., 2011), whereas the inhibition of the insulin receptor limited synapse density (Dixon-Salazar et al., 2014). In this study, SRT2104, an activator of SIRT1, increased hippocampal dendritic lengths and dendritic spine densities. However, the inhibition of SIRT1 in diabetic rats using Sirtinol induced a decrease in dendritic lengths and spine densities. Michán et al. (2010) also found that cognitive deficits in SIRT1 knockout mice were associated with defects in dendritic branching, branch length, and the complexity of neuronal dendritic arbors. Thus, diabetes-induced brain insulin resistance may regulate the expression of SIRT1, which, in turn, affects the morphology of the dendritic spine and disrupts cognitive function. However, Sirtinol, which was used in this study, has also been reported to decrease cell viability by activating adenosine 5′monophosphate-activated protein kinase (Hsu et al., 2012). The up-regulation of adenosine 5′monophosphate-activated protein kinase can aggravate tau aggregation and result in neuronal atrophy and spine loss in Alzheimer's disease (Liu et al., 2017). Because Sirtinol might also interact with other proteins in the cell, whether the effects of SIRT1 on the hippocampal structure of diabetic model rats are direct or indirect remain unclear. Thus, other methods, such as viral-mediated gene transfer, should be explored to further clarify the effects of SIRT1 on dendritic structures. In addition, a SIRT1 agonist was found to reduce the cognitive dysfunction in diabetic rats through antioxidative and anti inflammatory mechanisms (Wang et al., 2019). The effects of insulin signaling on improvements in mitochondrial oxidative metabolism and decreased inflammation have been researched in recent years (Dandona et al., 2007). Therefore, brain insulin signaling and SIRT1 may participate in the development of diabetes-related cognitive dysfunction through multiple processes, which are worth continuing to study in the future.
Under normal conditions, SIRT1 activates TORC1, which is a brain-specific modulator of CREB activity (Jeong et al., 2011). The maintenance of the hippocampal dendritic structure depends on the levels of BDNF, which can be transcribed by CREB (Sen, 2019). Because BDNF is thought to play a key role in the neuroprotective functions of SIRT1 (Jeong et al., 2011), we focused on TORC1/CREB signaling as a potential target pathway for the functions of SIRT1 in the regulation of dendritic spine morphology. We found that SIRT1 affects the morphology of dendritic spines and increases the expression of BDNF by modulating the TORC1/CREB signaling pathway in the hippocampus of diabetic model rats with cognitive dysfunction. However, we did not demonstrate the direct contribution of SIRT1 to dendritic morphology. Previous studies have demonstrated that SIRT1 directly deacetylates heat shock factor 1, which is involved in the maintenance of the total dendritic length, the density of dendritic spines, and the number of dendrites in pyramidal neurons of the hippocampus (Westerheide et al., 2009; Uchida et al., 2011). Extracellular regulated kinase1/2 may be another potential target molecule for SIRT1 during the modulation of dendritic morphology (Abe-Higuchi et al., 2016). Thus, SIRT1 may play diverse roles in reversing aberrant dendritic morphogenesis by regulating downstream signaling pathways. Future studies will be necessary to elucidate the mechanisms of SIRT1 regulation on dendritic spine morphology in diabetic rats.
In conclusion, the results of the present study suggested that brain insulin resistance is involved in the cognitive impairment of diabetic model rats through the activation of SIRT1 signaling. BDNF may be a downstream molecule of SIRT1 signaling, affecting the morphology of dendritic spines in the hippocampus of diabetic rats.
Footnotes
C-Editor: Zhao M; S-Editor: Yu J; L-Editors: Giles L, Song LP; T-Editor: Jia Y
Conflicts of interest:The authors declare no conflict of interest in this research.
Financial support:This study was supported by the National Natural Science Foundation of China, No. 81874464 (to YHW); the Natural Science Foundation of Hunan Province of China, No. 2019JJ50464 (to HY), and the Open Fund of the Domestic First-class Discipline Construction Project of Chinese Medicine of Hunan University of Chinese Medicine, No. 2018ZYX46 (to HY). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:The study was approved by the Animal Ethics Welfare Committee of the First Affiliated Hospital of Hunan University of Chinese Medicine (ZYFY201811207) in November 2018.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Funding:This study was supported by the National Natural Science Foundation of China, No. 81874464 (to YHW); the Natural Science Foundation of Hunan Province of China, No. 2019JJ50464 (to HY), and the Open Fund of the Domestic First-class Discipline Construction Project of Chinese Medicine of Hunan University of Chinese Medicine, No. 2018ZYX46 (to HY).
References
- 1.Abe-Higuchi N, Uchida S, Yamagata H, Higuchi F, Hobara T, Hara K, Kobayashi A, Watanabe Y. Hippocampal sirtuin 1 signaling mediates depression-like behavior. Biol Psychiatry. 2016;80:815–826. doi: 10.1016/j.biopsych.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhutada P, Mundhada Y, Humane V, Rahigude A, Deshmukh P, Latad S, Jain K. Agmatine, an endogenous ligand of imidazoline receptor protects against memory impairment and biochemical alterations in streptozotocin-induced diabetic rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:96–105. doi: 10.1016/j.pnpbp.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Boros BD, Greathouse KM, Gearing M, Herskowitz JH. Dendritic spine remodeling accompanies Alzheimer's disease pathology and genetic susceptibility in cognitively normal aging. Neurobiol Aging. 2019;73:92–103. doi: 10.1016/j.neurobiolaging.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. J Vis Exp. 2011:2920. doi: 10.3791/2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Candasamy M, Mohamed Elhassan SA, Kumar Bhattamisra S, Hua WY, Sern LM, Binti Busthamin NA, Mohamad Ilni NB, Shun NS, Baohong L, Ya NS, Ying NW. Type 3 diabetes (Alzheimer's disease): new insight for promising therapeutic avenues. Panminerva Med. 2020;62:155–163. doi: 10.23736/S0031-0808.20.03879-3. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y, Yan Z, Zhou T, Wang G. SIRT1 Regulates cognitive performance and ability of learning and memory in diabetic and nondiabetic models. J Diabetes Res. 2017;2017:7121827. doi: 10.1155/2017/7121827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Y, Jiang X, Ma H, Wang Y, Xue P, Liu Y. SIRT1 and insulin resistance. J Diabetes Complications. 2016;30:178–183. doi: 10.1016/j.jdiacomp.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58:708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi J, Ko J, Racz B, Burette A, Lee JR, Kim S, Na M, Lee HW, Kim K, Weinberg RJ, Kim E. Regulation of dendritic spine morphogenesis by insulin receptor substrate, a downstream effector of Rac1 and Cdc42 small GTPases. J Neurosci. 2005;25:869–879. doi: 10.1523/JNEUROSCI.3212-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dandona P, Chaudhuri A, Mohanty P, Ghanim H. Anti-inflammatory effects of insulin. Curr Opin Clin Nutr Metab Care. 2007;10:511–517. doi: 10.1097/MCO.0b013e3281e38774. [DOI] [PubMed] [Google Scholar]
- 12.Dixon-Salazar TJ, Fourgeaud L, Tyler CM, Poole JR, Park JJ, Boulanger LM. MHC class I limits hippocampal synapse density by inhibiting neuronal insulin receptor signaling. J Neurosci. 2014;34:11844–11856. doi: 10.1523/JNEUROSCI.4642-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan F, Qi J, Wang W, Liu N, Liu H, Xu X, Wang X, Tu Y, Wang W, Fu J. Amelioration of prediabetes-induced changes of dendritic structural plasticity. Front Biosci (Landmark Ed) 2019;24:291–302. doi: 10.2741/4718. [DOI] [PubMed] [Google Scholar]
- 14.Hamer JA, Testani D, Mansur RB, Lee Y, Subramaniapillai M, McIntyre RS. Brain insulin resistance: a treatment target for cognitive impairment and anhedonia in depression. Exp Neurol. 2019;315:1–8. doi: 10.1016/j.expneurol.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Hsu YF, Sheu JR, Lin CH, Yang DS, Hsiao G, Ou G, Chiu PT, Huang YH, Kuo WH, Hsu MJ. Trichostatin A and sirtinol suppressed survivin expression through AMPK and p38MAPK in HT29 colon cancer cells. Biochim Biophys Acta. 2012;1820:104–115. doi: 10.1016/j.bbagen.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Jeong H, Cohen DE, Cui L, Supinski A, Savas JN, Mazzulli JR, Yates JR, 3rd, Bordone L, Guarente L, Krainc D. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med. 2011;18:159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamal A, Biessels GJ, Urban IJ, Gispen WH. Hippocampal synaptic plasticity in streptozotocin-diabetic rats: impairment of long-term potentiation and facilitation of long-term depression. Neuroscience. 1999;90:737–745. doi: 10.1016/s0306-4522(98)00485-0. [DOI] [PubMed] [Google Scholar]
- 18.Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63:2232–2243. doi: 10.2337/db14-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CC, Huang CC, Hsu KS. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology. 2011;61:867–879. doi: 10.1016/j.neuropharm.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 21.Leszek J, Trypka E, Tarasov VV, Ashraf GM, Aliev G. Type 3 diabetes mellitus: a novel implication of alzheimers disease. Curr Top Med Chem. 2017;17:1331–1335. doi: 10.2174/1568026617666170103163403. [DOI] [PubMed] [Google Scholar]
- 22.Li XN, Chen L, Luo B, Li X, Wang CY, Zou W, Zhang P, You Y, Tang XQ. Hydrogen sulfide attenuates chronic restrain stress-induced cognitive impairment by upreglulation of Sirt1 in hippocampus. Oncotarget. 2017;8:100396–100410. doi: 10.18632/oncotarget.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D, Tang H, Li XY, Deng MF, Wei N, Wang X, Zhou YF, Wang DQ, Fu P, Wang JZ, Hébert SS, Chen JG, Lu Y, Zhu LQ. Targeting the HDAC2/HNF-4A/miR-101b/AMPK pathway rescues tauopathy and dendritic abnormalities in Alzheimer's disease. Mol Ther. 2017;25:752–764. doi: 10.1016/j.ymthe.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Yao Z, Zhang L, Zhu H, Deng W, Qin C. Insulin induces neurite outgrowth via SIRT1 in SH-SY5Y cells. Neuroscience. 2013;238:371–380. doi: 10.1016/j.neuroscience.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Luu L, Dai FF, Prentice KJ, Huang X, Hardy AB, Hansen JB, Liu Y, Joseph JW, Wheeler MB. The loss of Sirt1 in mouse pancreatic beta cells impairs insulin secretion by disrupting glucose sensing. Diabetologia. 2013;56:2010–2020. doi: 10.1007/s00125-013-2946-5. [DOI] [PubMed] [Google Scholar]
- 26.Machholz E, Mulder G, Ruiz C, Corning BF, Pritchett-Corning KR. Manual restraint and common compound administration routes in mice and rats. J Vis Exp. 2012:2771. doi: 10.3791/2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marampon F, Gravina GL, Festuccia C, Popov VM, Colapietro A, Sanità P, Musio D, De Felice F, Lenzi A, Jannini EA, Di Cesare E, Tombolini V. Vitamin D protects endothelial cells from irradiation-induced senescence and apoptosis by modulating MAPK/SirT1 axis. J Endocrinol Invest. 2016;39:411–422. doi: 10.1007/s40618-015-0381-9. [DOI] [PubMed] [Google Scholar]
- 28.Meijer RI, Barrett EJ. The insulin receptor mediates insulin's early plasma clearance by liver, muscle, and kidney. Biomedicines. 2021;9:37. doi: 10.3390/biomedicines9010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michán S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris RGM. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12:239–260. [Google Scholar]
- 31.Niyomchan A, Watcharasit P, Visitnonthachai D, Homkajorn B, Thiantanawat A, Satayavivad J. Insulin attenuates arsenic-induced neurite outgrowth impairments by activating the PI3K/Akt/SIRT1 signaling pathway. Toxicol Lett. 2015;236:138–144. doi: 10.1016/j.toxlet.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Pchitskaya EI, Zhemkov VA, Bezprozvanny IB. Dynamic microtubules in Alzheimer's disease: association with dendritic spine pathology. Biochemistry (Mosc) 2018;83:1068–1074. doi: 10.1134/S0006297918090080. [DOI] [PubMed] [Google Scholar]
- 33.Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajasekar N, Nath C, Hanif K, Shukla R. Intranasal insulin administration ameliorates streptozotocin (icv)-induced insulin receptor, neuroinflammation, amyloidogenesis, and memory impairment in rats. Mol Neurobiol. 2017;54:6507–6522. doi: 10.1007/s12035-016-0169-8. [DOI] [PubMed] [Google Scholar]
- 35.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 36.Rorbach-Dolata A, Piwowar A. Neurometabolic evidence supporting the hypothesis of increased incidence of type 3 diabetes mellitus in the 21st century. Biomed Res Int. 2019;2019:1435276. doi: 10.1155/2019/1435276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruegsegger GN, Vanderboom PM, Dasari S, Klaus KA, Kabiraj P, McCarthy CB, Lucchinetti CF, Nair KS. Exercise and metformin counteract altered mitochondrial function in the insulin-resistant brain. JCI Insight. 2019;4:e130681. doi: 10.1172/jci.insight.130681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott M, Flaherty D, Currall J. Statistics: general linear models (a flexible approach) J Small Anim Pract. 2014;55:527–530. doi: 10.1111/jsap.12260. [DOI] [PubMed] [Google Scholar]
- 39.Sen N. ER, CREB, and memory: a tangled emerging link in disease. Neuroscientist. 2019;25:420–433. doi: 10.1177/1073858418816611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shan Y, Zhang S, Gao B, Liang S, Zhang H, Yu X, Zhao J, Ye L, Yang Q, Shang W. Adipose tissue SIRT1 regulates insulin sensitizing and anti-inflammatory effects of berberine. Front Pharmacol. 2020;11:591227. doi: 10.3389/fphar.2020.591227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma P, Kumar S. S961, a biosynthetic insulin receptor antagonist, downregulates insulin receptor expression & suppresses the growth of breast cancer cells. Indian J Med Res. 2018;147:545–551. doi: 10.4103/ijmr.IJMR_403_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen T, Xu B, Lei T, Chen L, Zhang C, Ni Z. Sitagliptin reduces insulin resistance and improves rat liver steatosis via the SIRT1/AMPKα pathway. Exp Ther Med. 2018;16:3121–3128. doi: 10.3892/etm.2018.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spinelli M, Fusco S, Grassi C. Brain insulin resistance and hippocampal plasticity: mechanisms and biomarkers of cognitive decline. Front Neurosci. 2019;13:788. doi: 10.3389/fnins.2019.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sukhov IB, Lebedeva MF, Zakharova IO, Derkach KV, Bayunova LV, Zorina, Avrova NF, Shpakov AO. Intranasal administration of insulin and gangliosides improves spatial memory in rats with neonatal type 2 diabetes mellitus. Bull Exp Biol Med. 2020;168:317–320. doi: 10.1007/s10517-020-04699-8. [DOI] [PubMed] [Google Scholar]
- 45.Sun JL, Park J, Lee T, Jeong JH, Jung TW. DEL-1 ameliorates high-fat diet-induced insulin resistance in mouse skeletal muscle through SIRT1/SERCA2-mediated ER stress suppression. Biochem Pharmacol. 2020;171:113730. doi: 10.1016/j.bcp.2019.113730. [DOI] [PubMed] [Google Scholar]
- 46.Tabassum R, Jeong NY, Jung J. Protective effect of hydrogen sulfide on oxidative stress-induced neurodegenerative diseases. Neural Regen Res. 2020;15:232–241. doi: 10.4103/1673-5374.265543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torres MD, Garcia O, Tang C, Busciglio J. Dendritic spine pathology and thrombospondin-1 deficits in Down syndrome. Free Radic Biol Med. 2018;114:10–14. doi: 10.1016/j.freeradbiomed.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uchida S, Hara K, Kobayashi A, Fujimoto M, Otsuki K, Yamagata H, Hobara T, Abe N, Higuchi F, Shibata T, Hasegawa S, Kida S, Nakai A, Watanabe Y. Impaired hippocampal spinogenesis and neurogenesis and altered affective behavior in mice lacking heat shock factor 1. Proc Natl Acad Sci U S A. 2011;108:1681–1686. doi: 10.1073/pnas.1016424108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valdecantos MP, Pérez-Matute P, González-Muniesa P, Prieto-Hontoria PL, Moreno-Aliaga MJ, Martínez JA. Lipoic acid improves mitochondrial function in nonalcoholic steatosis through the stimulation of sirtuin 1 and sirtuin 3. Obesity (Silver Spring) 2012;20:1974–1983. doi: 10.1038/oby.2012.32. [DOI] [PubMed] [Google Scholar]
- 50.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang F, Shang Y, Zhang R, Gao X, Zeng Q. A SIRT1 agonist reduces cognitive decline in type 2 diabetic rats through antioxidative and anti inflammatory mechanisms. Mol Med Rep. 2019;19:1040–1048. doi: 10.3892/mmr.2018.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang H, Li W, Meng P, Liu Z, Liu J, Wang Y. Chronic unpredictable mild stress aggravates mood, cognitive impairment, and brain insulin resistance in diabetic rat. Evid Based Complement Alternat Med. 2018;2018:2901863. doi: 10.1155/2018/2901863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye T, Meng X, Zhai Y, Xie W, Wang R, Sun G, Sun X. Gastrodin ameliorates cognitive dysfunction in diabetes rat model via the suppression of endoplasmic reticulum stress and NLRP3 inflammasome activation. Front Pharmacol. 2018;9:1346. doi: 10.3389/fphar.2018.01346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng Y, Zhang L, Hu Z. Cerebral, insulin signaling pathway, and brain angiogenesis. Neurol Sci. 2016;37:9–16. doi: 10.1007/s10072-015-2386-8. [DOI] [PubMed] [Google Scholar]
- 57.Zhang LY. Mechanism of action of energy metabolism molecule SIRT1 in improving bone metabolism of type 2 diabetes. Zhongguo Zuzhi Gongcheng Yanjiu. 2020;24:276–281. [Google Scholar]
- 58.Zhang Y, Thai K, Jin T, Woo M, Gilbert RE. SIRT1 activation attenuates α cell hyperplasia, hyperglucagonaemia and hyperglycaemia in STZ-diabetic mice. Sci Rep. 2018;8:13972. doi: 10.1038/s41598-018-32351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]