King discusses work from the Machesky laboratory that identifies CYRI-A as a novel regulator of macropinocytosis that resolves macropinosome formation by locally sequestering active RAC1.
Abstract
In this issue, Le et al. (2021. J. Cell Biol. https://doi.org/10.1083/jcb.202012114) describe a new role for the recently discovered protein CYRI in controlling the protrusions that allow cells to engulf extracellular fluid by macropinocytosis. This study helps explain how these structures are disassembled, but also uncovers a new mechanism linking the ability of cells to drink and their capacity for invasive migration.
The actin cytoskeleton is the major determinant of cellular mechanics, generating and shaping the protrusions that fulfill a wide range of cellular functions. Many of these require dynamic regulation over both space and time, and therefore a diverse array of proteins control almost every aspect of actin biology.
One of the newest members of the actin-regulator club is CYFIP-related RAC1 interacting (CYRI) protein (also known as FAM49). Rather than regulating actin biochemistry directly, CYRI mimics and therefore competes with binding of the Scar/Wiskott Aldrich Syndrome Family verprolin homologous protein (WAVE) complex to its upstream activator Rac1 (1). This provides a mechanism to locally suppress Scar/WAVE activation and actin nucleation, which is important to prevent excessively stable protrusions and even the hijacking of ruffling used by invasive pathogens such as Salmonella to enter host cells (2).
Although human cells contain two closely related CYRI paralogues (CYRI-A and -B), previous studies focused entirely on CYRI-B. The localization and dynamics of either protein was also unknown due lack of reliable antibodies and difficulties tagging either end of the protein. In this issue, Le and colleagues perform the first investigation of CYRI-A and solve the localization question by introducing internal GFP tags, allowing them to observe CYRI dynamics for the first time (3).
Unexpectedly, the most striking localization of both CYRI paralogues was to macropinocytic structures. Macropinocytosis is an endocytic process whereby ruffles or cup-shaped protrusions at the plasma membrane fold in on themselves to form large vesicles filled with extracellular fluid. Macropinocytosis is highly regulated and only occurs at a low rate in most unstimulated cells but can dramatically increase in cancers, allowing them to use extracellular proteins for food. This is the ancient conserved role also used by amoebae, whereas other cells such as those of the immune system have developed more specialized uses for fluid uptake in antigen surveillance and cell migration (4).
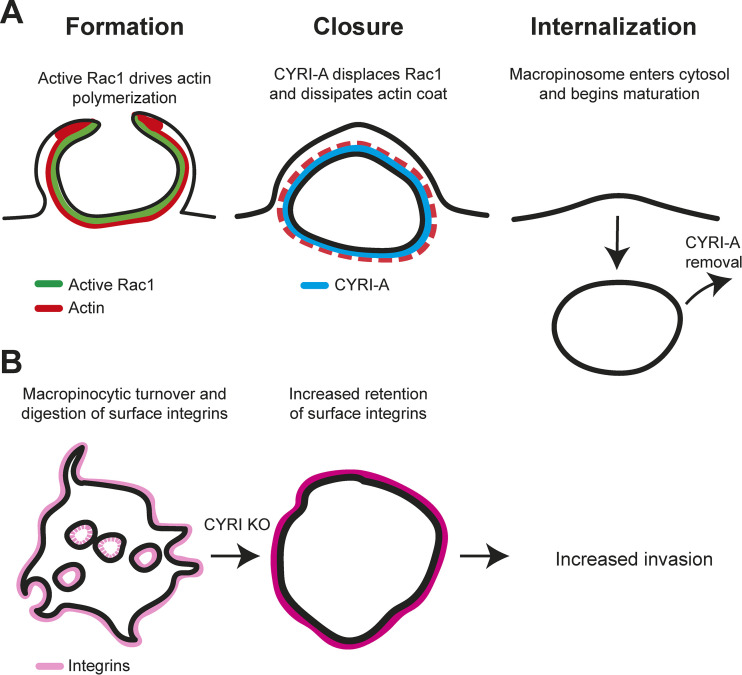
Like lamellipodia, macropinocytic protrusions are also driven by the Scar/WAVE complex. However, fluid capture requires additional regulation to both shape protrusions so they efficiently capture fluid and then dismantle them upon closure to allow newly formed vesicles to move into the cytosol (Fig. 1 A; reviewed in 5). Le et al. found that CYRI-A was strongly recruited as macropinocytic cups closed, coincident with loss of active Rac1 and their actin coat but preceding the Rab5 delivery that marks the first steps in intracellular processing. Consistent with this, fluid uptake is significantly reduced in CYRI-deficient cells.
Figure 1.
CYRI-A regulates macropinosome formation and integrin turnover. (A) Macropinocytic protrusions are generated by localized actin polymerization, regulated by Rac1. CYRI-A is recruited by active Rac1 as cups close, driving actin disassembly and movement of the newly formed vesicle into the cytosol. (B) In addition to other endocytic pathways, macropinocytosis makes a significant contribution to the internalization and subsequent degradation of surface proteins such as integrins. Blocking macropinocytosis by disruption of CYRI prevents this, leading to elevation of integrins at the cell surface, enhancing invasive migration.
Surprisingly, while CYRI-A and B can functionally compensate for each other during migration, they localize differently: CYRI-A is recruited to cups as a brief burst around macropinosome closure, whereas CYRI-B is more uniform at the plasma membrane and on later, more tubulated macropinocytic vesicles. This raises the possibility that the CYRIs may play overlapping but subtly different roles, which may be important in cell types where they are differentially expressed.
An additional crucial finding by Le et al. is that macropinocytosis significantly contributes to integrin adhesion receptor turnover at the plasma membrane (3). The internalization and subsequent trafficking of surface proteins is key for regulating the cell surface composition and therefore function and responses to extracellular signals. Surface proteins and receptors are primarily internalized by classical pathways such as clathrin-mediated endocytosis. These account for the vast majority of surface turnover, and the presence of clathrin and adapter protein coats at the site of internalization allows specific proteins or activated receptors to be selectively captured and regulated. In contrast, macropinocytic structures are relatively inefficient at internalizing membrane and appear to lack any comparable coat or adaptors. Macropinocytosis is therefore largely considered a minor and nonselective route to internalize surface proteins.
While integrin internalization occurs through multiple pathways, the authors found that macropinocytosis makes a significant contribution. Blocking macropinocytosis led to retention and elevation of integrins at the cell surface, thereby enhancing invasive migration (Fig. 1 B). As macropinocytosis is elevated in many cancers, this contribution to surface proteome turnover may prove as clinically important as its well-established role in nutrient acquisition.
Macropinocytic internalization of surface proteins may also be much more selective than previously thought. While not directly addressed in their study, data from Le et al. indicates that integrins may even be specifically enriched at macropinocytic cups. Another recent study also showed enrichment of β1-integrin at macropinocytic ruffles, as well as adhesion complex components such as talin and vinculin (6). Crucially, the receptor tyrosine kinase AXL was also enriched, but not N-cadherin—providing the first clear evidence of selectivity. If receptors can be enriched at macropinocytic structures, then their ligands can be too. It therefore follows that there may be selective removal of extracellular proteins/ligands by macropinocytosis, such as the integrin-mediated uptake of extracellular matrix previously shown to help support cell growth under starvation (7).
How macropinocytic selectivity is achieved remains an open question, although it is likely the actin cytoskeleton plays a central role. The fate and signaling potential of proteins internalized by macropinocytosis is also very poorly understood. Surface proteins can be retrieved from macropinosomes via the WASP and Scar homologue and retromer sorting complexes (8), and captured soluble components such as albumin can be rescued by specific receptors before degradation (9). However, the selectivity and subsequent trafficking of recovered proteins remains almost completely unexplored. Understanding how macropinosomes internalize specific proteins and where they go next will be essential in understanding their impact in both cancer biology and the normal physiology of constitutively macropinocytic cells such as macrophages and dendritic cells.
Using an elegant combination of cell biology, biochemistry, and microscopy, Le et al. define a new role for the CYRI proteins in macropinocytosis and demonstrate a new route by which it can modify cancer biology in addition to simply providing nutrients. The shared role for CYRI in both macropinocytic and migratory structures underlines their shared evolutionary origins, but whether CYRI affects other processes or Rac1 effectors other than Scar/WAVE remains unknown. The suppression of small GTPase signaling by mimicking an effector also represents a regulatory mechanism potentially shared by other Rho-family members in other contexts. These details will be determined in due course, but this study represents an important step forward in our understanding of both CYRI function and the dynamic regulation of actin during macropinocytosis.
Acknowledgments
J.S. King is funded by Royal Society University Research Fellowship URF\R\201036.
The author declares no competing financial interests.
References
- 1.Fort, L., et al. 2018. Nat. Cell Biol. 10.1038/s41556-018-0198-9 [DOI] [Google Scholar]
- 2.Yuki, K.E., et al. 2019. Nat. Microbiol. 10.1038/s41564-019-0484-8 [DOI] [PubMed] [Google Scholar]
- 3.Le, A.H., et al. 2021. J. Cell Biol. 10.1083/jcb.202012114 [DOI] [Google Scholar]
- 4.King, J.S., et al. 2019. Philos. Trans. R. Soc. Lond. B Biol. Sci. 10.1098/rstb.2018.0158 [DOI] [Google Scholar]
- 5.Mylvaganam, S., et al. 2021. Curr. Biol. 10.1016/j.cub.2021.01.036 [DOI] [PubMed] [Google Scholar]
- 6.Zdżalik-Bielecka, D., et al. 2021. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.2024596118 [DOI] [Google Scholar]
- 7.Muranen, T., et al. 2017. Nat. Commun. 10.1038/ncomms13989 [DOI] [Google Scholar]
- 8.Buckley, C.M., et al. 2016. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.1524532113 [DOI] [Google Scholar]
- 9.Toh, W.H., et al. 2019. J. Cell Sci. 10.1242/jcs.235416 [DOI] [PubMed] [Google Scholar]