Abstract
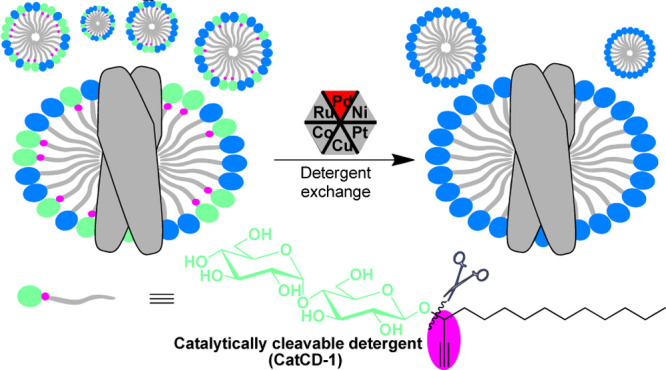
Throughout the in vitro studies of membrane proteins (MPs), proper detergents are essential for the preparation of stable aqueous samples. To date, universally applicable detergents have not yet been reported to accommodate the distinct requirements for the highly diversified MPs and at the different stages of MP manipulation. Detergent exchange often has to be performed. We report herein the catalytically cleavable detergents (CatCDs) that can be efficiently removed to facilitate a complete exchange. To this end, functional groups, like propargyl and allyl, are introduced as branched chains or built in the hydrophobic chain close to the hydrophilic head. The representative CatCDs can be used as usual detergents in the extraction and purification of MPs and later be removed upon the addition of catalytic palladium. Mediated by CatCD-1, reconstitution of a transporter protein MsbA into a series of detergents was achieved. The extension of these designs could facilitate the future optimization of other biophysics studies.
Introduction
Membrane proteins (MPs) are fundamentally important biological molecules and are thus popular translational drug targets.1 They currently account for more than 50% of FDA-approved drugs for the treatment of many human diseases.2 Nevertheless, the high-resolution structure of MPs has been a great challenge to explore. Owing to the amphipathic nature of MPs, their manipulation and characterization require a proper membrane-like environment.3−6 Throughout in vivo studies, detergents are the most common reagents from which micelles are assembled in water to accommodate MPs. Despite the ever-expanding detergent repertoire, such as tripod amphiphiles,7,8 neopentyl glycol series,9−14 facial amphiphiles,15,16 peptide detergents,17−19 and amphipols,20−22 universal detergents for topologically divergent MPs, as well as for various biophysical and biochemical studies, remain unavailable. In line with these different requirements and sometimes with the cost issue, detergent exchange is often the critical step during sample preparation.22,23
Current protocols for detergent exchange are to physically lower the concentration of the original detergent below its critical micelle concentration (CMC) by dilution, dialysis, or adsorption using BioBeads.24,25 These approaches usually leave behind contaminant residual detergents, unless iterative steps are applied. However, susceptible MPs cannot survive. In addition, probably due to the unspecific interaction between the polymer membrane surface and MPs, the current dialysis often leads to aggregation. Furthermore, traditional methods also uneconomically consume a large quantity of detergents, especially for detergents with a high CMC.
Alternatively, we have recently reported a chemical method enabled by a chemically cleavable detergent (CCD),26 which can be completely removed. However, the quantitative amount of the reductant required for the cleavage of the disulfide bond in the CCD possibly impairs MPs. Additionally, cleavable surfactants were previously reported, such as silane,27 acetal,28,29 acrylate,27 and azosulfonate30 compounds that could be cleaved by fluoride, acid, and photoirradiation. These surfactants greatly facilitated MS studies. However, few of them was applicable in this context. These detergents are generally too harsh to preserve the accurate native structures of MP during purification and stabilization. As a matter of fact, the silane detergent (ammonium as the hydrophilic head) and azosulfonate detergent would largely denature MPs. What is more, the cleavage conditions of these detergents are also too harsh for most MPs. For example, silane detergents were cleaved by KF solution at high concentration. Acrylate detergents were cleaved by long-time UV light irradiation. Overall, there is few tool detergents available for mediating efficient detergent exchange. Herein, we develop catalytically cleavable detergents (CatCDs) for the facilitation of bio-orthogonal removal, based on which we establish a new protocol for convenient MP reconstitution in various commercial detergents.
Results and Discussion
Design and Physical Properties of CatCDs
To design a cleavable detergent, a shooting point is embedded at the hydrophobic chain, which is close to the hydrophilic part where we had previously introduced a disulfide bond. To this end, propargyl and allyl groups were incorporated because both of them are well-known caging moieties that can be catalytically and bio-orthogonally decaged by using transition metals.33−38 Furthermore, these two groups are small, and remodeling with them can minimize the potentially adverse effects on the detergent assembly. On the template of a traditional detergent n-dodecyl-β-d-maltopyranoside (DDM), integrating the structural features of the propargyl or allyl group afforded six new detergents CatCD-1–6 (Figure 1). When ethynyl and vinyl groups are introduced at C1, the chain-branched type detergents CatCD-1 and CatCD-2 are obtained, respectively. Appending methylene at C2 yields another type of detergent CatCD-3. Meanwhile, by direct introduction of a triple bond onto the C2–C3 bond of the main chain, the linear detergent CatCD-4 is designed. Similarly, Z- and E-double bond modification on the C2–C3 bond results in linear detergents CatCD-5 and CatCD-6, respectively.
Figure 1.
Design of catalytically cleavable detergents (CatCDs).
All these new detergents are highly soluble in water (>10%, w/v) (Table 1). Their CMCs were measured by fluorophore encapsulation assay.39 The embedding of branched chains into the hydrophobic chain of DDM, like ethynyl and vinyl in CatCD-1 and CatCD-2, respectively, moderately decreases CMCs (0.10 and 0.06 mM, respectively, vs 0.17 mM for DDM). When compared to their counter partner Mal 12_2 (CMC ≈ 0.02 mM)40 with a similar two-carbon ethyl branch at the same location, this decrease of CMC values is not significant. Probably due to the different rigidity and orientation of the branch, the minimal difference (only the difference in the saturation from ethynyl and vinyl to ethyl) results in a drastic effect on the micelle formation. The methylene branch in CatCD-3 also exert only a negligible decrease in CMC (0.12 mM) when compared with DDM. This observation can be ascribed to the unfavorable packing of the rigid unsaturated side chains of these CatCDs. For the linear detergents CatCD-4–6 showing unsaturation in the main chain while maintaining the same carbon numbers, all CMC values increase compared to DDM (0.45, 0.26, and 0.20 mM for CatCD-4, -5, and -6, respectively), consistently reflecting the adverse effect of rigid unsaturation on micelle formation. Micelle sizes were measured through dynamic light scattering (DLS) analysis. All branched chain detergents (CatCD-1–3) form larger micelles (Dh ≈ 8 nm) than does DDM (Dh ≈ 6.8 nm), once again differentiating them from Mal 12_2, which formed even larger micelles (Dh ≈ 20 nm). By contrast, all linear detergents (CatCD-4–6) form slightly smaller micelles than does DDM.
Table 1. Physical Characterization of CatCDs.
| compds | MWa (g/mol) | CMCb (mM) | micelle sizec (Dh, nm) | Sol.d (m/v) |
|---|---|---|---|---|
| CatCD-1 | 534.6 | ∼0.10 | 8.0 ± 0.5 | >10% |
| CatCD-2 | 536.6 | ∼0.06 | 7.8 ± 0.1 | >10% |
| CatCD-3 | 522.6 | ∼0.12 | 8.3 ± 0.3 | >10% |
| CatCD-4 | 506.6 | ∼0.45 | 6.1 ± 0.2 | >10% |
| CatCD-5 | 508.6 | ∼0.26 | 6.3 ± 0.1 | >10% |
| CatCD-6 | 508.6 | ∼0.20 | 6.7 ± 0.1 | >10% |
| Mal 12_2e | 538.6 | 0.02e | ∼20e | N.A. |
| DDMf | 510.6 | ∼0.17 | 6.8 ± 0.1 | >20% |
Molecular weight.
CMC values were determined in ddH2O by the fluorophore encapsulation assay.39
The hydrodynamic diameters of micelles were calculated by DLS in ddH2O.
Solubility in ddH2O.
See ref (40).
See website: www.anatrace.com.
Thermal Stability of MPs in CatCDs
These detergents were then evaluated on the thermal stabilization of two different MPs. One is the ATP-binding cassette transporter MsbA, which catalyzes the movement of the core lipopolysaccharide across the inner membrane of bacteria.32,41 The other is the Class A G protein-coupled receptor, adenosine receptor 2A (A2AAR), which is an important drug target.42 The stability of these MPs in detergents was assessed with an established thermal stability assay for quality control in the crystallization study.43 Generally, the transition temperature of each sample in the test detergent reflects the thermal stabilization to the protein. For the purpose of this CPM assay, MsbA was engineered to introduce a buried cysteine residues A30C, while mutated two out-exposing cysteine residues C88A and C315A (Figure S1).31 Overall, these detergents have sufficient mildness in protecting MsbA from denaturation, except for CatCD-4 with alkyne in the main chain, which significantly attenuated the stability of MsbA (Figures 2A and S2) when compared with DDM and lauryl maltose neopentyl glycol (LMNG). The other two linear CatCDs with Z- and E-olefins in the main chain, that is, CatCD-5 and CatCD-6, are also inferior to DDM. Branched chain CatCDs are generally comparable to DDM in terms of MsbA stabilization. The alkyne branched chain CatCD-1 and the methylene-embedded CatCD-3 even showed excellent stabilization comparable to LMNG, one of the most popular detergents to date. For A2AAR, similar results were obtained and CatCD-4 again performed worst (Figure 2A). To further evaluate these detergents, MsbA solutions in various detergents were heated at 40 °C for 10 min followed by analysis with analytical size-exclusion chromatography (aSEC). MsbA in CatCD-1, -2, and -3 could largely maintain nonaggregated, while MsbA in CatCD-4, -5, and -6 showed significant aggregation under this condition (Figure 2B).
Figure 2.

Stability of MPs in various detergents. (A) Thermal stability of MsbA (blue) and A2AAR (green) in each detergent (CatCDs, DDM, and LMNG). The transition temperature (Tm, °C) was calculated from the CPM profiling of MsbA in the test detergent at CMC + 0.2% (w/v) concentration. Error bars represent SEM, n = 4 for MsbA and n = 2 for A2AAR. (B) SEC profiling of MsbA in detergents after heating at 40 °C for 10 min.
Screening Conditions for Chemical Cleavage of CatCDs
These CatCDs were then investigated for their cleavages (Figure 3A). A total of 14 different transition metal species were tested (Figure 3A and Table S1). Using octyl glucoside as an internal standard, residual CatCDs were quantified using HPLC analysis with the detection using the evaporative light-scattering detector (ELSD) (Figure S3). Under standard conditions (10 mol %, 0.10 mM), ethynyl branched CatCD-1 stood out among the series. CatCD-1 could be more or less cleaved by the test metal species, except K2PtCl6. Among them, most palladium species favored the complete cleavage of CatCD-1, probably via a Pd(II)-mediated hydration pathway.38,44 The catalyst Pd(dba)2 was the only one ineffective in the cleavage, probably because it was buried in the inner of the micelle due to its hydrophobicity. Some palladium catalysts also exhibited moderate activities on CatCD-4 with alkyne embedded in the main chain, likely indicative of the selectivity of palladium on the triple bond over the double bond. Indeed, all olefin detergents (CatCD-2, -3, -5, and -6) were inert toward all these transition metals, except CatCD-5, which also achieved cleavage by Pd(OAc)2 to some extent. The difference in reactivity between CatCD-1 and CatCD-4 suggested an important role of steric hindrance because the terminal alkyne in CatCD-1 was less hindered than the di-substituted alkyne in CatCD-4. Furthermore, the alkyne branched at C1 in CatCD-1 also seemed to be more accessible to the Pd catalyst than alkyne at C2–C3 in CatCD-4. Meanwhile, CatCD-1 was also partially cleaved by some other metal species, including RuCl3, K2PtCl4, CoCl2, Co2(CO)8, Ni(OAc)2, and Cu(OAc)2. Intriguingly, these metals also moderately catalyzed the cleavage of another branch-type detergent CatCD-2, while retaining other detergents. The reactivity difference between branched (CatCD-1 and -2) and others (CatCD-3, -4, -5, and -6) again corroborated the substitution sensitivity of this reaction. In other words, the outperformance of CatCD-1 in this screening exploited its less hindered branched alkyne, which was more reactive toward palladium species.
Figure 3.

Screening and optimization of reaction conditions for the CatCD cleavage. (A) Heatmap plotting of transition metal-mediated detergent decomposition, as detected by ELSD–HPLC (100% represents complete cleavage). CatCDs (1.0 mM in ddH2O) were treated with each transition metal (10 mol %, 0.10 mM) at room temperature for 8 h. (B) Cleavage of CatCD-1 by different palladium species at various loadings. (C) Cleavage of CatCD-1 by [Pd(allyl)Cl]2 and Pd(OAc)2 in different buffers.
In view of its stabilization of MPs and ready cleavability, CatCD-1 was therefore picked up for further evaluation on its pairing palladium catalysts (Figure 3B). With decreased loading (5 mol %, 0.05 mM), these catalysts remained effective, especially Pd(OAc)2 and [Pd(allyl)Cl]2, by which less than 5% of CatCD-1 remained after treatment for 8 h. Finally, [Pd(allyl)Cl]2 stuck out when the catalyst amount was further decreased (2 mol %, 0.02 mM). Nearly 80% of CatCD-1 decomposed under this condition. More importantly, the cleavage mediated by [Pd(allyl)Cl]2 was consistently effective in common buffers, whereas the cleavage by Pd(OAc)2 was effective only in PBS buffer (Figure 3C). Tris or HEPES buffer probably poisoned the palladium catalyst through the undesired ligand exchange. In fact, cleavage by [Pd(allyl)Cl]2 (5 mol %, 0.05 mM) was very fast in PBS buffer that more than 50% of CatCD-1 was cleaved within 5 min and more than 95% after 2 h (Figure S4). Interestingly but unexpectedly, the cleavage of CatCD-1 in organic solvents was ineffective. Actually, about 60% cleavage of CatCD-1 in CH3OH was observed, while in THF, the cleavage was hardly observable (Figure S5), indicating possible promotion by the micellar effect under the aqueous condition. CatCD-1 by [Pd(allyl)Cl]2 (5 mol %, 0.05 mM) was smoothly cleaved in the presence of typical detergents, including neutral glycosides and PEG derivatives, as well as zwitterionic CHAPS and amino N-oxide (Figure S6). Meanwhile, under this optimized condition, MsbA remained intact (Figure S7). Taken together, CatCD-1 demonstrated its excellent bio-orthogonality that could facilitate in situ detergent exchange.
Detergent Screening for the Stabilization of MsbA
With the ever-increasing availability of detergents, high or median throughput screening is required to select a detergent for a given MP because the bottom-up preparation of the MP sample in a single detergent is inefficient and costly. Mediated by CatCD-1, we performed herein a complete detergent exchange for detergent-screening studies for MsbA (Figure 4A). Initially, MsbA was solubilized in CatCD-1, which was comparable to that in the common detergents LMNG and undecyl maltoside (UDM) in terms of yield and monodispersity (Figure 4B). MsbA was then diluted into a plethora of representative detergents (Table S2). Notably, in considering the rapidity of the cleavage reaction, the concentration of each test detergent was set at 10× CMC (or CMC + 0.2% for detergents with high CMC values, such as CHAPS, NG, and LDAO), at which the test detergent could sufficiently cover the decomposed detergent in time and prevent MP aggregation due to the slow diffusion of the detergent in and between micelles. Treatment of these samples with [Pd(allyl)Cl]2 (5 mol %, 0.025 mM) tracelessly removed CatCD-1 and afforded MsbA samples in test detergents (Figure 4C). In maltoside detergents such as LMNG, DDM, UDM, and decyl maltoside, MsbA exhibited good monodispersity in aSEC. Other neutral detergent, like TX-100, also maintained MsbA monomer well. Only NG were inferior in the maintenance of monodispersity for MsbA. Conversely, MsbA reconstituted into zwitterionic detergents such as LDAO and CHAPS showed completely aggregated peaks. This ranking of detergent preference for MsbA was consistent to previous screening results, confirming that this CatCD-Pd-mediated detergent exchange could be a reliable and convenient approach to MP reconstitution. In comparison, when the previously reported CCD-TCEP system was applied, MsbA completely aggregated (Figure S8).
Figure 4.

Reconstitution of MsbA in various detergents mediated by CatCD-1. (A) Scheme of MsbA sampling from solubilization and detergent exchange. (B) aSEC profiling of MsbA solubilized by using CatCD-1, UDM, and LMNG. (C) aSEC profiling of MsbA in detergents exchanged from CatCD-1.
Conclusions
In summary, we developed CatCD-1, based on which a protocol of efficient detergent exchange was established and exemplified through MsbA sampling. The reaction conditions are subject to further optimization in terms of catalyst loading, temperature, pH, and so forth. Given the high selectivity of the terminal propargyl group over other unsaturated bonds in the main chain, this protocol should be amenable to natural lipid systems with or without the presence of olefins and thus be applicable for nanodisc preparation. Encouraged by this biocompatible reaction condition, other transition metal-mediated detergents or lipid modification can also be expected by introducing bond formation chemistry, in addition to a bond-breaking one.
Experimental Section
Synthesis and Characterization of CatCDs
CatCDs were synthesized by glycosylation of the commercially available peracetylated maltoside with appropriately appended aliphatic alcohols. By a scalable two-step procedure, (CatCD-1-6) were obtained in a total yield of 22–75% (Scheme S1). NMR (nuclear magnetic resonance) characterization was carried out on a Bruker AVANCE III 500 or 800 spectrometer (FT, 500/800 MHz for 1H NMR; 126/201 MHz for 13C NMR) at 298 K with CDCl3 or CD3OD as the solvent using tetramethylsilane as the internal standard. HRMS (high-resolution mass spectra) were recorded on an Agilent 6230 mass spectrometer using electrospray ionization. Synthetic procedures, characterization data, and NMR spectra of all CatCDs are given in detail in the Supporting Information. Reagents and solvents were from commercial suppliers (Adamas, Anatrace, Accela, Aladdin, J&K, Sigma, etc.) and used without further purification.
CMC Determination by the Fluorophore Encapsulation Assay
ANS (8-aniline-1-naphthyl ammonium sulfonate) is an environment-dependent fluorescence dye (377/466 nm). Its fluorescence becomes high in a hydrophobic environment. Accordingly, the CMC of the detergent can be determined. Detergent solutions appended with ANS (10 μM) were prepared by series dilution in a 96-well plate. The plate was incubated at room temperature for 1 h before the fluorescence intensity was measured at 405/455 nm using a multimode plate reader. The CMC value of the test detergent was obtained as the turning point of concentration in which fluorescence intensity changed sharply. The measurement was repeated in three parallels for each detergent.
Micelle Size Measurement by DLS
The detergent solutions (10 mM in ddH2O) were filtered through a 0.2 μm filter. The hydrodynamic diameter of the micelles produced by CatCDs or DDM was measured by using the DLS instrument in three parallels. The hydrodynamic diameter (Dh) of detergent micelles was calculated according to the Stokes–Einstein relation and other related physical formulas. The temperature was kept at 25 °C throughout all the measurements.
Protein Expression and Purification
The engineered A2AAR-BRIL-ΔC construct was expressed in Spodoptera frugiperda (Sf 9) cells.31,43 Purification: after homogenization, the cell membrane was washed with low-salt buffer for two times and then with high-salt buffer (1 M NaCl, 10 mM MgCl2, 20 mM KCl, 20 mM HEPES buffer, pH 7.5, 30% glycerol) for three times. The membrane sediment was resuspended into low-salt buffer after centrifugation, and iodoacetamide was added and mixed at 4 °C for 1 h. The cell membrane in an equal volume of 2× solubilization buffer [100 mM HEPES 7.5, 1.6 M NaCl, 2% (w/v) DDM, 0.4% (w/v) cholesteryl hemisuccinate (CHS)] followed by incubation for 2.5 h at 4 °C. After high-speed centrifugation, the supernatant was collected and incubated with the TALON IMAC resin (Clontech) at 4 °C overnight in the presence of 20 mM imidazole. The resin bound with MPs was sequentially washed with 10-column volumes of wash I buffer [50 mM HEPES 7.5, 800 mM NaCl, 10% (w/v) glycerol, 0.1% (w/v) DDM, 0.02% (w/v) CHS, 20 mM imidazole, 10 mM MgCl2, 8 mM ATP, antagonist 40 μM ZM241385] and 5-column volumes of wash II buffer [25 mM HEPES 7.5, 800 mM NaCl, 10% (w/v) glycerol, 0.05% (w/v) DDM, 0.01% (w/v) CHS, 50 mM imidazole, 40 μM ZM241385]. The desired MP was then collected by subjecting three-column volumes of elution buffer [25 mM HEPES 7.5, 800 mM NaCl, 10% (w/v) glycerol, 0.025% (w/v) DDM, 0.005% (w/v) CHS, 220 mM imidazole, and 40 μM ZM241385]. The protein concentration was measured using a Pierce BCA protein detection kit. The protein status was evaluated using the SDS PAGE gel assay and SEC curves. The purified protein was stored in the −80 °C refrigerator for thermal stability assays.
MsbA including an N-terminal 6×-His tag is expressed in Escherichia coli.32 Three mutations were introduced to the construct: A30C, C88A, and C315A. Purification: after homogenization, the cell membrane was washed with low-salt buffer for two times and then with high-salt buffer (1 M NaCl, 10 mM MgCl2, 20 mM KCl, 20 mM HEPES buffer, pH 7.5) for three times. The membrane sediment was resuspended into low-salt buffer after centrifugation, and iodoacetamide was added and mixed at 4 °C for 1 h. The cell membrane in an equal volume of 2× solubilization buffer (100 mM HEPES 7.5, 200 mM NaCl, 1% w/v LMNG or 0.5% w/v CatCD-1, 0.4% w/v CHS) was incubated for 2.5 h at 4 °C. The supernatant was collected after high-speed centrifugation and incubated with the TALON IMAC resin (Clontech) at 4 °C overnight in the presence of 20 mM imidazole. Wash I buffer: 20 mM HEPES 7.5, 100 mM NaCl, 0.01% w/v LMNG or 0.1% w/v CatCD-1, 20 mM imidazole. Wash II buffer: 20 mM HEPES 7.5, 100 mM NaCl, 0.01% w/v LMNG or 0.1% w/v CatCD-1, 50 mM imidazole. Elute buffer: 20 mM HEPES 7.5, 100 mM NaCl, 300 mM imidazole, 0.005% w/v LMNG or 1 mM CatCD-1. The protein concentration was measured using a Pierce BCA protein detection kit. The protein status was evaluated using the SDS PAGE gel assay and SEC curves. The purified protein was stored in the −80 °C refrigerator for the thermal stability assays and detergent reconstitution experiment.
Procedures for Thermal Shift Assay
Each sample was prepared by mixing 2.5 μL of MP (containing about 0.5 μg of MsbA in LMNG or 0.5 μg of A2AAR in DDM) with 22.5 μL of test detergent buffer (500 mM NaCl, 2% glycerol, 25 mM HEPES, 0.2% + CMC (w/v) test detergent). Samples were incubated on ice (about at 4 °C) for 30 min. The CPM dye (N-([4-(7-diethylamino-4-methyl-3-coumarinyl) phenyl]-maleimide) was diluted into each sample in a final concentration of 2 μg/mL, followed by additional 10 min incubation at 4 °C. All samples were subjected to thermal denaturation with a ramp rate of 1 °C/min from 25 to 90 °C in a Rotor-Gene Q spectrofluorometer (Qiagen). With the denaturation, the exposed cysteine reacts with the CPM dye and turns on the fluorescence by the adduct (λex = 365 nm and λem = 460 nm). Data were processed using the GraphPad Prism program. Tm values were obtained by fitting data with Boltzmann sigmoidal.
Procedures for Detergent Removal and Screening
25 μL of MsbA (∼0.2 mg/mL) in CatCD-1 (2 mM) was added to 25 μL of detergent buffer (500 mM NaCl, 10% glycerol, 20× CMC detergent or CMC + 0.2%, 20 mM PBS, pH 7.5) and incubated on ice (about at 4 °C) for 1 h. [Pd(allyl)Cl]2 (0.05 mM in DMSO) was then added, and the abovementioned mixture was incubated for another 2 h before being centrifuged at 12,000 rpm for 10 min. The homogeneity of all MsbA samples was then evaluated through analytical size exclusion chromatography (aSEC, HPLC: Agilent, Bioinert 1100 series; column: Nanofilm SEC-250, 4.6 × 250 mm, 5 μm, Sepax Technologies Inc.), flow speed: 0.5 mL/min with SEC buffer (25 mM HEPES, pH 7.5, 500 mM NaCl, 0.05% DDM, 0.01% cholesterol hemisuccinate, 2% glycerol), and temperature: 10 °C. Profiles were measured by a 280 nm UV absorbance detector.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (no. 21672147 to H.T.). We thank Q. Shi, J. Liu, Q. Tan, and L. Yang for assistance in the membrane preparation and NMR processing at the Core Facility of iHuman Institute, ShanghaiTech University. We thank D. Liu (ShanghaiTech University), J. Hou (Fudan University), and G. Song (East China Normal University) for insightful discussions and assistance in manuscript preparation.
Glossary
Abbreviations
- MP
membrane protein
- CatCD
catalytically cleavable detergent
- DDM
n-dodecyl-β-d-maltopyranoside
- GPCR
G protein-coupled receptors
- NMR
nuclear magnetic resonance
- HRMS
high-resolution mass spectra
- A2AAR
A2A adenosine receptor
- CPM
N-([4-(7-diethylamino-4-methyl-3-coumarinyl) phenyl] maleimide
- CMC
critical micelle concentration
- ELSD
evaporative light scattering detector
- aSEC
analytical size-exclusion chromatography
- DLS
dynamic light scattering
- LMNG
lauryl maltose neopentyl glycol
- OG
n-octyl-β-d-glucopyranoside
- UDM
n-undecyl-β-d-maltopyranoside
- DM
n-decyl-β-d-maltopyranoside
- NG
n-nonyl-β-d-glucopyranoside
- LDAO
n-dodecyl-N,N-dimethylamine-N-oxide
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c02894.
Positions of the A30C mutation site of MsbA; thermal stability of MPs in CatCDs, DDM, and LMNG; evaluation of CatCD cleavage in different metal catalysts; evaluation of CatCD-1 cleavage in different palladium catalysts detected by ELSD–HPLC; time course of CatCD-1 cleavage mediated by [Pd(allyl)Cl]2 in three different buffer solutions; evaluation of CatCD-1 cleavage in organic solvents detected by ELSD–HPLC; cleavage of CatCD-1 in the presence of typical detergents; SEC profiling of MsbA in the treatment of [Pd(allyl)Cl]2; SEC profiling of MsbA before and after detergents exchanged in CCD-2 or CatCD-1; commercial detergents for reconstitution studies of MsbA; general methods for synthetic chemistry; and NMR spectra and purity characterization (PDF)
Author Contributions
∥ L.L. and Z.Z. contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Overington J. P.; Al-Lazikani B.; Hopkins A. L. Opinion - How many drug targets are there?. Nat. Rev. Drug Discovery 2006, 5, 993–996. 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Santos R.; Ursu O.; Gaulton A.; Bento A. P.; Donadi R. S.; Bologa C. G.; Karlsson A.; Al-Lazikani B.; Hersey A.; Oprea T. I.; Overington J. P. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discovery 2017, 16, 19–34. 10.1038/nrd.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. A.; Liu J. J.; Chun E.; Wacker D.; Wu H.; Cherezov V.; Stevens R. C. GPCR stabilization using the bicelle-like architecture of mixed sterol-detergent micelles. Methods 2011, 55, 310–317. 10.1016/j.ymeth.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.-X.; Cross T. A. Influences of membrane mimetic environments on membrane protein structures. Annu. Rev. Biophys. 2013, 42, 361–392. 10.1146/annurev-biophys-083012-130326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loll P. J. Membrane protein structural biology: the high throughput challenge. J. Struct. Biol. 2003, 142, 144–153. 10.1016/s1047-8477(03)00045-5. [DOI] [PubMed] [Google Scholar]
- Marty M. T.; Hoi K. K.; Robinson C. V. Interfacing membrane mimetics with mass spectrometry. Acc. Chem. Res. 2016, 49, 2459–2467. 10.1021/acs.accounts.6b00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae P. S.; Laible P. D.; Gellman S. H. Tripod amphiphiles for membrane protein manipulation. Mol. Biosyst. 2010, 6, 89–94. 10.1039/b915162c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae P. S.; Kruse A. C.; Gotfryd K.; Rana R. R.; Cho K. H.; Rasmussen S. G. F.; Bae H. E.; Chandra R.; Gether U.; Guan L.; Kobilka B. K.; Loland C. J.; Byrne B.; Gellman S. H. Novel tripod amphiphiles for membrane protein analysis. Chem.—Eur. J. 2013, 19, 15645–15651. 10.1002/chem.201301423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae P. S.; Rasmussen S. G. F.; Rana R. R.; Gotfryd K.; Chandra R.; Goren M. A.; Kruse A. C.; Nurva S.; Loland C. J.; Pierre Y.; Drew D.; Popot J.-L.; Picot D.; Fox B. G.; Guan L.; Gether U.; Byrne B.; Kobilka B.; Gellman S. H. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat. Methods 2010, 7, 1003–1008. 10.1038/nmeth.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. H.; Byrne B.; Chae P. S. Hemifluorinated maltose-neopentyl glycol (HF-MNG) amphiphiles for membrane protein stabilisation. Chembiochem 2013, 14, 452–455. 10.1002/cbic.201200759. [DOI] [PubMed] [Google Scholar]
- Chae P. S.; Rana R. R.; Gotfryd K.; Rasmussen S. G. F.; Kruse A. C.; Cho K. H.; Capaldi S.; Carlsson E.; Kobilka B.; Loland C. J.; Gether U.; Banerjee S.; Byrne B.; Lee J. K.; Gellman S. H. Glucose-neopentyl glycol (GNG) amphiphiles for membrane protein study. Chem. Commun. 2013, 49, 2287–2289. 10.1039/c2cc36844g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. H.; Husri M.; Amin A.; Gotfryd K.; Lee H. J.; Go J.; Kim J. W.; Loland C. J.; Guan L.; Byrne B.; Chae P. S. Maltose neopentyl glycol-3 (MNG-3) analogues for membrane protein study. Analyst 2015, 140, 3157–3163. 10.1039/c5an00240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H. E.; Cecchetti C.; Du Y.; Katsube S.; Mortensen J. S.; Huang W.; Rehan S.; Lee H. J.; Loland C. J.; Guan L.; Kobilka B. K.; Byrne B.; Chae P. S. Pendant-bearing glucose-neopentyl glycol (P-GNG) amphiphiles for membrane protein manipulation: Importance of detergent pendant chain for protein stabilization. Acta Biomater. 2020, 112, 250–261. 10.1016/j.actbio.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H. E.; Du Y.; Hariharan P.; Mortensen J. S.; Kumar K. K.; Ha B.; Das M.; Lee H. S.; Loland C. J.; Guan L.; Kobilka B. K.; Chae P. S. Asymmetric maltose neopentyl glycol amphiphiles for a membrane protein study: effect of detergent asymmetricity on protein stability. Chem. Sci. 2019, 10, 1107–1116. 10.1039/c8sc02560f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C.; Bennett B. C.; Hong W.-X.; Fu Y.; Baker K. A.; Marcoux J.; Robinson C. V.; Ward A. B.; Halpert J. R.; Stevens R. C.; Stout C. D.; Yeager M. J.; Zhang Q. Steroid-based facial amphiphiles for stabilization and crystallization of membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, E1203–E1211. 10.1073/pnas.1221442110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Ma X.; Ward A.; Hong W.-X.; Jaakola V.-P.; Stevens R. C.; Finn M. G.; Chang G. Designing facial amphiphiles for the stabilization of integral membrane proteins. Angew. Chem., Int. Ed. Engl. 2007, 46, 7023–7025. 10.1002/anie.200701556. [DOI] [PubMed] [Google Scholar]
- Tao H.; Lee S. C.; Moeller A.; Roy R. S.; Siu F. Y.; Zimmermann J.; Stevens R. C.; Potter C. S.; Carragher B.; Zhang Q. Engineered nanostructured beta-sheet peptides protect membrane proteins. Nat. Methods 2013, 10, 759–761. 10.1038/nmeth.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P.; Zhao X.; Vaughn M.; Baldo M. A.; Bruce B. D.; Zhang S. Self-assembling peptide detergents stabilize isolated photosystem I on a dry surface for an extended time. PLoS Biol. 2005, 3, e230 10.1371/journal.pbio.0030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor C.-L.; Chen L.; Pomroy N. C.; Hwang P.; Go S.; Chakrabartty A.; Privé G. G. Lipopeptide detergents designed for the structural study of membrane proteins. Nat. Biotechnol. 2003, 21, 171–176. 10.1038/nbt776. [DOI] [PubMed] [Google Scholar]
- Tribet C.; Audebert R.; Popot J.-L. Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 15047–15050. 10.1073/pnas.93.26.15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popot J.-L.; Althoff T.; Bagnard D.; Banères J.-L.; Bazzacco P.; Billon-Denis E.; Catoire L. J.; Champeil P.; Charvolin D.; Cocco M. J.; Crémel G.; Dahmane T.; de la Maza L. M.; Ebel C.; Gabel F.; Giusti F.; Gohon Y.; Goormaghtigh E.; Guittet E.; Kleinschmidt J. H.; Kühlbrandt W.; Le Bon C.; Martinez K. L.; Picard M.; Pucci B.; Sachs J. N.; Tribet C.; van Heijenoort C.; Wien F.; Zito F.; Zoonens M. Amphipols from A to Z*. Annu. Rev. Biophys. 2011, 40, 379–408. 10.1146/annurev-biophys-042910-155219. [DOI] [PubMed] [Google Scholar]
- Tribet C.; Diab C.; Dahmane T.; Zoonens M.; Popot J.-L.; Winnik F. M. Thermodynamic characterization of the exchange of detergents and amphipols at the surfaces of integral membrane proteins. Langmuir 2009, 25, 12623–12634. 10.1021/la9018772. [DOI] [PubMed] [Google Scholar]
- Ritchie T. K.; Grinkova Y. V.; Bayburt T. H.; Denisov I. G.; Zolnerciks J. K.; Atkins W. M.; Sligar S. G. Chapter 11-Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 2009, 464, 211–231. 10.1016/s0076-6879(09)64011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsan M.; Du Y.; Scull N. J.; Tikhonova E.; Tarrasch J.; Mortensen J. S.; Loland C. J.; Skiniotis G.; Guan L.; Byrne B.; Kobilka B. K.; Chae P. S. Highly branched pentasaccharide-bearing amphiphiles for membrane protein studies. J. Am. Chem. Soc. 2016, 138, 3789–3796. 10.1021/jacs.5b13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M.; Du Y.; Ribeiro O.; Hariharan P.; Mortensen J. S.; Patra D.; Skiniotis G.; Loland C. J.; Guan L.; Kobilka B. K.; Byrne B.; Chae P. S. Conformationally preorganized diastereomeric norbornane-based maltosides for membrane protein study: Implications of detergent kink for micellar properties. J. Am. Chem. Soc. 2017, 139, 3072–3081. 10.1021/jacs.6b11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue D.; Wang J.; Song X.; Wang W.; Hu T.; Ye L.; Liu Y.; Zhou Q.; Zhou F.; Jiang Z.-X.; Liu Z.-J.; Tao H. A chemical strategy for amphiphile replacement in membrane protein research. Langmuir 2019, 35, 4319–4327. 10.1021/acs.langmuir.8b04072. [DOI] [PubMed] [Google Scholar]
- Norris J. L.; Hangauer M. J.; Porter N. A.; Caprioli R. M. Nonacid cleavable detergents applied to MALDI mass spectrometry profiling of whole cells. J. Mass Spectrom. 2005, 40, 1319–1326. 10.1002/jms.914. [DOI] [PubMed] [Google Scholar]
- Yu Y.-Q.; Gilar M.; Lee P. J.; Bouvier E. S. P.; Gebler J. C. Enzyme-friendly, mass spectrometry-compatible surfactant for in-solution enzymatic digestion of proteins. Anal. Chem. 2003, 75, 6023–6028. 10.1021/ac0346196. [DOI] [PubMed] [Google Scholar]
- Norris J. L.; Porter N. A.; Caprioli R. M. Mass spectrometry of intracellular and membrane proteins using cleavable detergents. Anal. Chem. 2003, 75, 6642–6647. 10.1021/ac034802z. [DOI] [PubMed] [Google Scholar]
- Brown K. A.; Chen B.; Guardado-Alvarez T. M.; Lin Z.; Hwang L.; Ayaz-Guner S.; Jin S.; Ge Y. A photocleavable surfactant for top-down proteomics. Nat. Methods 2019, 16, 417–420. 10.1038/s41592-019-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W.; Weber E.; Geisse S.; Memmert K. Optimisation of protein expression and establishment of the wave bioreactor for baculovirus/insect cell culture. Cytotechnology 2002, 38, 77–85. 10.1023/a:1021102015070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A.; Reyes C. L.; Yu J.; Roth C. B.; Chang G. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 19005–19010. 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusop R. M.; Unciti-Broceta A.; Johansson E. M. V.; Sánchez-Martín R. M.; Bradley M. Palladium-mediated intracellular chemistry. Nat. Chem. 2011, 3, 239–243. 10.1038/nchem.981. [DOI] [PubMed] [Google Scholar]
- Streu C.; Meggers E. Ruthenium-induced allylcarbamate cleavage in living cells. Angew. Chem., Int. Ed. Engl. 2006, 45, 5645–5648. 10.1002/anie.200601752. [DOI] [PubMed] [Google Scholar]
- Li J.; Yu J.; Zhao J.; Wang J.; Zheng S.; Lin S.; Chen L.; Yang M.; Jia S.; Zhang X.; Chen P. R. Palladium-triggered deprotection chemistry for protein activation in living cells. Nat. Chem. 2014, 6, 352–361. 10.1038/nchem.1887. [DOI] [PubMed] [Google Scholar]
- Li J.; Chen P. R. Development and application of bond cleavage reactions in bioorthogonal chemistry. Nat. Chem. Biol. 2016, 12, 129–137. 10.1038/nchembio.2024. [DOI] [PubMed] [Google Scholar]
- Wang X.; Liu Y.; Fan X.; Wang J.; Ngai W. S. C.; Zhang H.; Li J.; Zhang G.; Lin J.; Chen P. R. Copper-triggered bioorthogonal cleavage reactions for reversible protein and cell surface modifications. J. Am. Chem. Soc. 2019, 141, 17133–17141. 10.1021/jacs.9b05833. [DOI] [PubMed] [Google Scholar]
- Latocheski E.; Dal Forno G. M.; Oliveira B. L.; Bernardes G. J. L.; Domingos J. B. Mechanistic insights into transition metal-mediated bioorthogonal uncaging reactions. Chem. Soc. Rev. 2020, 49, 7710–7729. 10.1039/d0cs00630k. [DOI] [PubMed] [Google Scholar]
- De Vendittis E.; Palumbo G.; Parlato G.; Bocchini V. A fluorimetric method for the estimation of the critical micelle concentration of surfactants. Anal. Biochem. 1981, 115, 278–286. 10.1016/0003-2697(81)90006-3. [DOI] [PubMed] [Google Scholar]
- Hong W.-X.; Baker K. A.; Ma X.; Stevens R. C.; Yeager M.; Zhang Q. Design, synthesis, and properties of branch-chained maltoside detergents for stabilization and crystallization of integral membrane proteins: human connexin 26. Langmuir 2010, 26, 8690–8696. 10.1021/la904893d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayatti P. S.; Lee S. C.; Stanfield R. L.; Wen P.-C.; Tajkhorshid E.; Wilson I. A.; Zhang Q. Structural insights into the lipid A transport pathway in MsbA. Structure 2019, 27, 1114–1123.e3. 10.1016/j.str.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Chun E.; Thompson A. A.; Chubukov P.; Xu F.; Katritch V.; Han G. W.; Roth C. B.; Heitman L. H.; IJzerman A. P.; Cherezov V.; Stevens R. C. Structural basis for allosteric regulation of GPCRs by sodium ions. Science 2012, 337, 232–236. 10.1126/science.1219218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov A. I.; Mileni M.; Chien E. Y. T.; Hanson M. A.; Stevens R. C. Microscale fluorescent thermal stability assay for membrane proteins. Structure 2008, 16, 351–359. 10.1016/j.str.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Santra M.; Ko S.-K.; Shin I.; Ahn K. H. Fluorescent detection of palladium species with an O-propargylated fluorescein. Chem. Commun. 2010, 46, 3964–3966. 10.1039/c001922d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



