Abstract

Sex pheromone analogues were synthesized and tested on two pest carposinid moth species: the guava moth, Coscinoptycha improbana, and the raspberry bud moth, Heterocrossa rubophaga. The pheromone analogues used for the electroantennogram testing included (Z)-11-methylenenonadec-7-ene, (Z)-nonadec-12-en-9-amine, (Z)-11-methoxynonadec-7-ene, (Z)-1-(octylsulfinyl)-dec-3-ene, and (Z)-nonadec-12-en-9-ol. An imine analogue, N-((Z)-nonadec-12-en-9-ylidene)cyclopropanamine, was also synthesized but was too unstable for testing with the moths. None of the analogue compounds elicited significant responses from the male moth antennae.
Introduction
Carposinid or “fruitworm” moths, as they are more commonly known, are economic pests in Asia and New Zealand.1−3 The fruitworm name originates from the internal feeding behavior of the larval stage, which causes spoilage of fruits, while also making them difficult to control with traditional pesticides. Hence, there is a need for new, effective pest management tools for these pest moths.
The three main pest species, Carposina sasakii (peach fruit moth), Heterocrossa rubophaga (raspberry bud moth), and Coscinoptycha improbana (guava moth), all had sex pheromones identified.3−6 The pheromone blends of these three species all contain (7Z)-alken-11-ones, while the raspberry bud moth and guava moth pheromone blends also contain the alkene (Z)-tricos-7-ene. The commonality of the functional group locations in this family is unusual. The majority of moth pheromones identified across other families are predominantly Type I and Type II pheromones where sympatric species use variations in the location and type of functional groups to achieve species isolation.7 For example, the well-studied Tortricidae family of moths use Type I pheromones (aliphatic acetates, aldehydes, and primary alcohols) typically with a principal carbon chain length of 12, 14, or 16 carbons. The tortricids vary the location(s), number, and geometry of the double bonds to form a vast array of pheromone components for achieving species isolation.8 On the other hand, we believe the commonality of pheromone structures within the Carposinidae family offers a unique opportunity for multiple species disruption with a single pheromone analogue.
Pheromone analogues have been well reported for Type I and Type II moth pheromones, and that is starting to carry over into those of the less studied families.9 For the carposinid moths, Suckling et al. investigated chain length variants using the peach fruit moth pheromone to disrupt guava moth.10 More recently, Twidle et al. have shown that the ester analogue (Z)-heptyl undec-4-enoate has an inhibitory effect on raspberry bud moth,11 similar to that of the formate analogues of Type I aldehyde pheromones.12 The aim of this work was to build on that knowledge and investigate changes directly to the carbonyl group of (7Z)-alken-11-ones of the carposinids, focusing on (Z)-nonadec-12-en-9-one (1) (or as it is historically known, (7Z)-nonadecen-11-one) which is common to the three known carposinid pheromone blends.
Results and Discussion
Synthesis
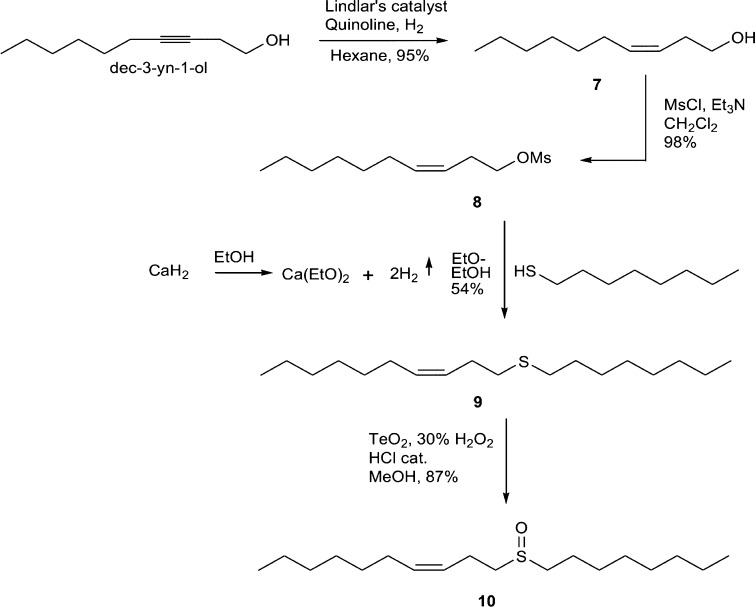
Starting with the previously prepared pheromone component (Z)-nonadec-12-en-9-one (1),11 a series of functional group interconversions were attempted (Scheme 1). This began with the methylene analogue, (Z)-11-methylenenonadec-7-ene (2), which was prepared via Wittig olefination. Methyltriphenylphosphonium iodide was converted to the ylide using potassium hexamethyldisilazide as the base prior to the addition of 1. The reaction proceeded well and after purification gave (Z)-11-methylenenonadec-7-ene (2) in 63% yield. (Z)-Nonadec-12-en-9-amine (3) was prepared in moderate yield (33%) from 1 via reductive amination using sodium cyanoborohydride. Imine (Z)-N-(nonadec-12-en-9-ylidene)cyclopropanamine (4) was prepared, also starting from 1. Analysis by gas chromatography–mass spectrometry (GC–MS) showed m/z 319 [M]+ representing the desired molecular ion. Upon exposure to moisture from the atmosphere, fast hydrolysis occurred. Due to this instability under ambient conditions, no further testing of the imine (4) was conducted since it did not represent a viable option as a pest management tool. Attempts were also made to prepare the thione, (Z)-nonadec-12-ene-9-thione, from the corresponding ketone using Lawesson reagent. However, multiple attempts of the reaction under various conditions proved unsuccessful. Investigation in the literature showed that Camps et al. had experienced similar problems with Lawesson reagent when trying to prepare moth pheromone-like structures and had to resort to more complicated methods, so attempts to form the thione were abandoned.13
Scheme 1. Synthesis of Analogues via Functional Group Interconversions.
In a similar fashion to the functional group transformations listed above, the methyl ether analogue, (Z)-11-methoxynonadec-7-ene (6), was prepared from the alcohol precursor of the pheromone,11 (Z)-nonadec-12-en-9-ol (5) (Scheme 2). The reaction performed best when methyl iodide was freshly prepared via the reaction of methanol and concentrated hydroiodic acid in water.14 The alcohol was first treated with NaH followed by methyl iodide, and the reaction proceeded well, giving (Z)-11- methoxynonadec-7-ene (6) in a 60% yield.
Scheme 2. Synthesis of Methyl Ether Analogue.
Synthesis of the sulfoxide analogue, (Z)-1-(octylsulfinyl)-dec-3-ene (10) (Scheme 3), began with the preparation of (Z)-dec-3-en-1-ol (7) from the related alkyne using Lindlar’s catalyst. This reaction proceeded extremely well giving alcohol (7) in an excellent 95% yield and high isomeric purity. Mesylation of alcohol (7) gave (Z)-dec-3-enyl methanesulfonate (8) in excellent yield of 98% prior to formation of thioether, (Z)-dec-3-enyl(octyl)sulfane (9). To prepare 9, calcium ethoxide was first prepared from CaH2 and ethanol and then used to deprotonate octylthiol for use in a Williamson etherlike synthesis. Rigorous degassing of the solvents reduced the unwanted disulfide formation, allowing the target compound (Z)-dec-3-enyl(octyl)sulfane (9) to be produced with a satisfactory yield of 54%. The final step of the synthesis was the oxidation of the thioether (9) to the target sulfoxide (Z)-1-(octylsulfinyl)-dec-3-ene (10). This was achieved using the TeO2–H2O2 system of Kwan et al.,15 giving the sulfoxide (10) in 87% yield.
Scheme 3. Synthesis of the Sulfoxide Analogue.
All of the tetrahedral analogues synthesized above were prepared as racemic mixtures for the antennal testing. Since the moths’ related pheromone component is planar at the carbonyl, we decided it was best to initially test racemic material and only explore absolute configurations where the compounds showed antennal activity.
EAG Testing
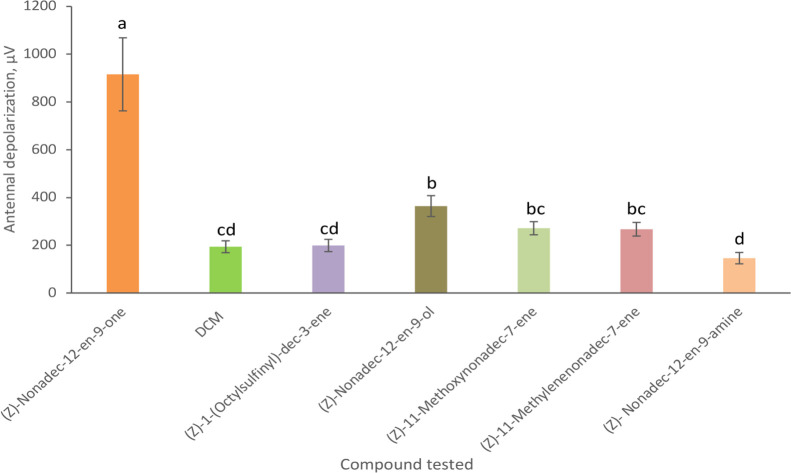
For the raspberry bud moth, only the pheromone component and the alcohol analogue elicited Electroantennogram (EAG) responses that were significantly different from the dichloromethane (DCM) control puff (Figure 1). Considering the earlier work of Twidle et al. where two of the ester analogues gave substantial antennal responses,11 we found it surprising that only one analogue was giving a very minor response. When the alcohol’s tetrahedral structure was considered, compared to the planar structure of the pheromone, it seemed unlikely that only this analogue would give a response. The methylene analogue (2) was planar like the pheromone, while the sulfoxide analogue (10) had an oxygen with a double bond, yet neither gave responses from the moth. Conversely, the methyl ether (6) and amine analogues (3) were tetrahedral structures similar to the alcohol analogue, yet gave no response. This led us to further investigate the alcohol analogue.
Figure 1.
Mean EAG responses (±SEM) of the male raspberry bud moth to 10 μg of the pheromone component (Z)-nonadec-12-en-9-one and pheromone analogues (n = 5). Bars that do not have the same letter are significantly different (Tukey 95% confidence intervals).
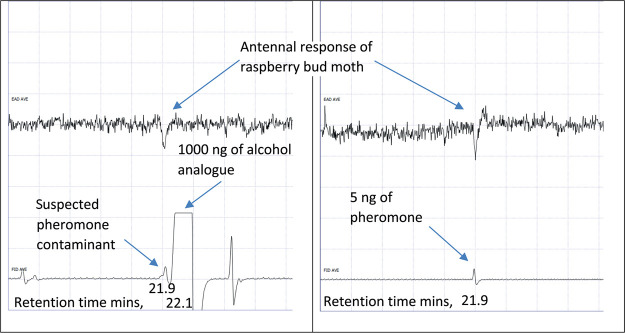
Since (Z)-nonadec-12-en-9-ol (5) had not been freshly prepared (it was a sample prepared from a previous trial ca. 2 years ago), it seemed possible that a small amount of the alcohol may have oxidized to the pheromone component (Z)-nonadec-12-en-9-one (1). However, initial GC–MS analysis of a dilute solution of the alcohol had shown no sign of the pheromone component. However, GC–electroantennogram detection (GC–EAD) testing with the concentrated alcohol analogue solution from the EAG experiments above showed a small antennal response from all moths tested to a small peak before the main alcohol peak (Figure 2). From comparison with a synthetic standard, the small peak was identified as the ketone pheromone component (1) (Figure 2). Based on these GC–EAD runs, the response seen from the raspberry bud moth to the alcohol analogue solution in previous EAG experiments were attributed to the small amount of the ketone pheromone present in the alcohol analogue solution. Hence, none of the analogues with changes at the carbonyl were biologically active with the raspberry bud moth.
Figure 2.
Example GC–EAD responses of the male raspberry bud moth (n = 3) to the alcohol analogue solution (left) and the pheromone component (right).
Guava moth responded similarly, only giving a weak response to the alcohol analogue. Responses from all other analogues were not significantly different from that of the control DCM puff (Figure 3). Again, GC–EAD analysis was run with the alcohol analogue solution from the EAG testing on the guava moth to isolate the response from that solution. Again, the small ketone peak before the alcohol analogue was observed to give a response from the guava moth male antenna (Figure 4).
Figure 3.
Mean EAG responses (±SEM) of the male guava moth to 10 μg of pheromone component (Z)-nonadec-12-en-9-one and pheromone analogues (n = 5). Bars that do not have the same letter are significantly different (Tukey 95% confidence intervals).
Figure 4.

Example GC–EAD responses of the male guava moth (n = 3) to the alcohol analogue solution.
The lack of response from the two moth species to the carbonyl analogues was both surprising and disappointing. The previous success of the ester analogues for this family from the work of Twidle et al. had been very encouraging. Here, (Z)-heptyl undec-4-enoate and (Z)-non-2-enyl nonanoate had given antennal responses from both moth species, while (Z)-heptyl undec-4-enoate had a strong inhibitory effect on the catch of male raspberry bud moth to pheromone lures in the field environment.11 It was hoped that this series of analogues would produce more potential pest management tools; unfortunately that was not the case. From comparison with other moth families, it is interesting that only the ester analogues gave responses for the carposinids. For the more widely studied tortricid and noctuid moth families, multiple functional groups have been found to be antennally active as analogues.16−18 For example, the pest tortricid, codling moth (Cydia pomonella), uses (8E,10E)-dodeca-8,10-dien-1-ol as its main pheromone component and responds to a range of analogues including geometric isomers, the related acetate and aldehyde, monoene C-12 alcohols, as well as halogenated analogues.16,17 These analogues for tortricid and noctuid moths are based on Type I moth pheromones that naturally show variation in the functional group (alcohol, acetate, and aldehyde), double-bond position (C-3 to C-13), and configuration (Z or E) since it is these variations that the moth species use for their reproductive isolation.8,19 For the few carposinid sex pheromones identified to date, there is no variation in the functional group or double-bond location and geometry, so perhaps it should be expected that a few analogues will be detected by these moths. This of course could change, and new opportunities arise as more is learnt about the reproductive behavior and pheromone chemistry of the wider carposinid family. We have chosen to report these negative results as they provide a useful record of the work done on a little studied moth family with an uncommon pheromone functional group while concomitantly preventing potential future duplication of work by other research groups.
Materials and Methods
General Procedures
These have been previously reported and are described briefly here.11 All NMR spectra were recorded on a 400 MHz Bruker Avance III spectrometer. Chemical shifts are reported relative to the solvent peak of chloroform and/or CDCl3 (δ 7.26 for 1H and δ 77.0 for 13C). 1H NMR data are reported as position (δ), relative integral, multiplicity (s, singlet; d, doublet; t, triplet; dt, double triplet; m, multiplet; br, broad peak), coupling constant (J, Hz), and the assignment of the atom. 13C NMR data are reported as position (δ) and assignment of the atom. NMR assignments were made using HSQC and HMBC data. A PerkinElmer Spectrum 1000 series Fourier transform IR spectrometer was used to record IR spectra where absorption maxima are expressed in wave numbers (cm–1). High-resolution mass spectra (HRMS) were recorded using electrospray ionization on a MicroTOF-Q mass spectrometer. All reaction products were analyzed on an Agilent 7890B gas chromatograph coupled to an Agilent 5977A mass selective detector. A DB-5ms column (30 m × 0.25 mm i.d. × 0.25 μm) was installed in the gas chromatograph, and samples were analyzed using previously described methods.20
Reactions were monitored using thin-layer chromatography (TLC) with premade silica gel plates from Sigma-Aldrich. Vanillin in acidified (H2SO4) ethanol was used as a developer solution for the TLC where heat was applied to develop the plate and reveal the spot/s.
Column chromatography was undertaken using standard 230 mesh silica gel (Sigma-Aldrich) on a medium pressure liquid chromatography system. The solvent/gradient systems used are outlined in the synthetic procedures below.
Chemicals
All chemical reagents were from Sigma-Aldrich (St. Louis, MO) and used in their supplied form unless otherwise stated. Solvents were dried over 3 Å molecular sieves, and ethers were distilled over LiAlH4 to remove the stabilizers before use. Methyl iodide (MeI) was prepared from the reaction of methanol and concentrated hydroiodic acid, with the MeI being distilled off and dried (MgSO4) as it formed.14 (Z)-Nonadec-12-en-9-one (1) and (Z)-nonadec-12-en-9-ol (5) were prepared using our previously reported methods.11 Briefly, these methods consisted of the acid catalyzed hydration of 2,3-dihydrofuran, followed by Wittig olefination favoring the cis double-bond geometry, giving (Z)-undec-4-en-1-ol. Dess–Martin oxidation of the alcohol gave the related aldehyde, and Grignard addition gave the desired alcohol (5). Further oxidation again using Dess–Martin periodinane gave the pheromone component (1).11
Synthetic Procedures
Synthesis details and compound characterizations are reported below, while NMR spectra can be found in the Supporting Information. Scheme 1 shows the functional group interconversions from the original pheromone component to the methylene, amine, and imine analogues. Schemes 2 and 3 show the synthetic routes used to make the methyl ether and sulfoxide analogues, respectively.
(Z)-11-Methylenenonadec-7-ene (2)
To a solution of methyltriphenylphosphonium bromide (714 mg, 2.00 mmol) in dry THF (10 mL), potassium hexamethyldisilazide solution (2.00 mL of 1.0 M in THF, 2.00 mmol) was added dropwise at room temperature under argon. The resulting solution was heated to reflux. After 2 h, the mixture was cooled to −80 °C, and (Z)-nonadec-12-en-9-one (1) (240 mg, 0.86 mmol) in dry THF (5 mL) was added dropwise. The mixture was allowed to warm to room temperature, and after 3 h, the reaction was quenched with 40 mL of NH4Cl (aq) solution. The organic layer was extracted with hexane (30 mL × 3) and dried (MgSO4). The solvent was removed, and the crude product was purified by column chromatography (SiO2, hexane/EtOAc) to give the title product (Z)-11-methylenenonadec-7-ene (2) (151 mg, 0.54 mmol, 63% yield, isomeric purity 94% by GC) as a clear colorless oil. δH (400 MHz; CDCl3; Me4Si) 0.88 (6H, t, J = 7.0 Hz, H-1, H-19), 1.23–1.43 (20H, m, H-2, H-3, H-4, H-5, H-13, H-14, H-15, H-16, H-17, H-18), 1.99–2.06 (6H, m, H-6, H-10, H-12), 2.14–2.19 (2H, m, H-9), 4.71 (1H, s, H-1′), 4.72 (1H, s, H-1′), 5.33–5.39 (2H, m, H-7, H-8). δC (100 MHz; CDCl3) 14.0, 14.1 (C-1, C-19), 22.6, 22.7 (C-2, C-18), 25.6 (C-9), 27.3 (C-6), 27.8 (C-13), 29.0, 29.3, 29.4, 29.5, 29.7 (C-4, C-5, C14, C15, C16), 31.8, 31.9 (C-3, C-17), 36.0, 36.1 (C-10, C-12), 108.7 (C-1′), 129.2 (C-8), 130.2 (C-7), 149.8 (C-11). IR: νmax(film)/cm–1; 2957, 2926, 2856, 1646, 1466, 1378, 889 and 737. MS data 70 eV, m/z (relative intensity) 278 (2, M+), 179 (66), 165 (62), 109 (37), 95 (77), 81 (100), 69 (49), 67 (71), 55 (58) and 41 (55).
(Z)-Nonadec-12-en-9-amine (3)
To a solution of ketone (1) (150 mg, 0.53 mmol) and NH4OAc (412 mg, 5.35 mmol) in dry MeOH (20 mL), NaBH3CN (34 mg, 0.54 mmol) was added. The mixture was stirred for 96 h after which time TLC showed the reaction was complete. MeOH was then removed under vacuum, and the resulting residue was taken up in HCl acidified water (pH < 2) and washed with EtOAc. The aqueous layer was then basified with NaOH (aq) (pH > 10) and extracted with DCM (20 mL × 3). The EtOAc and DCM extracts were combined and dried (MgSO4). The solvent was removed under vacuum, and the crude product was purified by column chromatography (silica gel, DCM/MeOH gradient) to give the title product (Z)-nonadec-12-en-9-amine (3) (50 mg, 0.18 mmol, 33% yield, isomeric purity >99% by GC) as a clear pale-yellow oil. δH (400 MHz; CDCl3; Me4Si) 0.86–0.90 (6H, m, H-1, H-19), 1.27–1.45 (22H, m, H-2, H-3, H-4, H-5, H-6, H-7, H-15, H-16, H-17, H-18, NH2), 1.66–1.80 (4H, m, H-8, H-10), 2.02–2.05 (2H, m, H-14), 2.14–2.22 (2H, m, H-11), 3.15 (1H, quin., J = 6.4 Hz, H-9), 5.28–5.34 (1H, m, H-12) 5.38–5.45 (1H, m, H-13). δC (100 MHz; CDCl3) 14.1 (C-1, C-19), 22.7, 22.7 (C-2, C18), 23.2 (C-11), 25.3 (C-7), 27.4 (C-14), 29.0, 29.3, 29.4, 29.7 (C-4, C-5, C-6, C-15, C-16) 31.8, 31.9 (C-3, C-17), 32.6, 32.7 (C-8, C-10), 52.2 (C-9), 127.3 (C-12), 131.7 (C-13). IR: νmax(film)/cm–1; 2957, 2923, 2855, 1612, 1519, 1459, 1379, 1116, and 722. MS data 70 eV, m/z (relative intensity) 281 (tr, M+), 264 (2), 210 (7), 196 (9), 182 (12), 168 (92), 142 (100), 69 (16), 55 (18), and 43 (21). HRMS (ESI+) found (MH+): 282.3153 C19H40N requires 282.3155.
(Z)-N-(Nonadec-12-en-9-ylidene)cyclopropanamine (4)
To a solution of ketone (1) (280 mg, 1.00 mmol) in dry ethanol (10 mL), cyclopropylamine (692 μL, 10.0 mmol) and acetic acid (5 drops) were added dropwise at room temperature under argon over 3 Å molecular sieves. The mixture was heated to reflux and left overnight. The product was not observed on TLC due to its instability, but GC–MS analysis confirmed the reaction had proceeded to completion. The product proved very unstable with a lifetime under 60 min in ambient conditions and hence was not taken forward for any other testing. MS data 70 eV, m/z (relative intensity) 319 (3, M+), 318 (5, M-1), 248 (100), 234 (78), 220 (43), 206 (51), 122 (40), 108 (36), 96 (45) and 41 (41).
(Z)-11-Methoxynonadec-7-ene (6)
To a solution of NaH (240 mg, 10 mmol) in THF (10 mL), (Z)-nonadec-12-en-9-ol (5) (230 mg, 0.81 mmol) in THF (5 mL) was added dropwise at room temperature under argon. The resulting mixture was stirred for 90 min after which time MeI (1 mL, 16.0 mmol) was added via a syringe. The reaction was monitored by TLC. After 17 h, more NaH (240 mg, 10.0 mmol) in THF (10 mL) was added, followed by a second addition of MeI (1 mL, 16.0 mmol). After 65 h, the reaction proceeded sufficiently and was quenched with 30 mL of MeOH and poured into brine (50 mL). The organics were extracted with Et2O (20 mL × 3), washed with brine (20 mL), and dried (MgSO4). The solvent was removed under vacuum, and the crude product was purified by column chromatography (silica gel, hexane/EtOAc gradient) to give the title product (Z)-11-methoxynonadec-7-ene (6) (146 mg, 0.49 mmol, 60% yield, isomeric purity >87% by GC) as a clear pale yellow oil. δH (400 MHz; CDCl3; Me4Si) 0.88 (6H, t, J = 6.8 Hz, H-1, H-19), 1.27–1.36 (20H, m, H-2, H-3, H-4, H-5, H-13, H-14, H-15, H-16, H-17, H-18), 1.43–1.52 (4H, m, H-10, H-12), 1.98–2.10 (4H, m, H-6, H-9), 3.11–3.17 (1H, m, H-11), 3.32 (3H, s, Me), 5.34–5.41 (2H, m, H-7, H-8). δC (100 MHz; CDCl3) 14.1 (C-1, C-19), 22.6, 22.7 (C-2, C18), 23.0 (C-9), 25.2 (C-13), 27.2 (C-6), 29.0, 29.3, 29.6, 29.7, 29.9 (C-4, C-5, C-14, C-15, C-16) 31.8, 31.9 (C-3, C-17), 33.3, 33.4 (C-10, C-12), 56.3 (Me), 80.4 (C-11), 129.4, 130.3 (C-7, C-8). IR: νmax(film)/cm–1; 2960, 2927, 2856, 1466, 1378, 1100, and 736. MS data 70 eV, m/z (relative intensity) 296 (tr, M+), 264 (45), 183 (36), 138 (33), 110 (50), 95 (70), 83 (75), 69 (100), 55 (64) and 41 (44). HRMS (ESI+) found (MNa+): 319.2971 C20H40NaO requires 319.2971.
(Z)-Dec-3-en-1-ol (7)
To a solution of dec-3-yn-1-ol (2.00 g, 13.0 mmol) in hexane (50 mL), Lindlar’s catalyst (280 mg) and quinoline (two drops) were added. The system was flushed with hydrogen and left for 90 min at room temperature after which time the reaction was confirmed as completed by GC–MS analysis of the reaction mixture. The catalyst was filtered off, and the organic layer was washed successively with 1 M HCl and water (20 mL each) and then dried (MgSO4). The solvent was removed under vacuum to afford the title product (Z)-dec-3-en-1-ol (7) (1.92 g, 12.3 mmol, 95% yield, isomeric purity 99% by GC) as a clear colorless oil which was used without further purification. δH (400 MHz; CDCl3; Me4Si) 0.88 (3H, t, J = 7.0 Hz, H-10), 1.27–1.39 (9H, m, H-6, H-7, H-8, H-9, OH), 2.07 (2H, dt, J = 7.0 and 7.2 Hz, H-5), 2.34 (2H, dt, J = 4.4 and 6.6 Hz, H-2), 3.62–3.66 (2H, m, H-1), 5.33–5.40 (1H, m, H-3), 5.53–5.60 (1H, m, H-4). The 1H NMR data matched that reported in the literature.21 MS data 70 eV, m/z (relative intensity) 156 (tr, M+), 138 (8, M-18), 110 (17), 109 (17), 95 (37), 81 (80), 68 (100), 67 (84), 55 (97) and 41 (68).
(Z)-Dec-3-enyl Methanesulfonate (8)
To a solution of (Z)-dec-3-en-1-ol (6) (1.00 g, 6.40 mmol) and Et3N (1782 μL, 12.8 mmol) in dry DCM (30 mL) under argon at 0 °C, mesyl chloride (986 μL, 12.7 mmol) was added dropwise with constant stirring. The mixture was stirred for 2 h and allowed to warm to room temperature. Upon completion, the reaction was quenched with water (10 mL) and was washed with 1 M HCl (10 mL × 2), aq. sat. NaHCO3 (20 mL), and brine (20 mL). The organic phase was dried (MgSO4), and the solvent was removed under vacuum to give the title product (Z)-dec-3-enyl methanesulfonate (8) (1.47 g, 6.26 mmol, 98% yield) as a clear yellow oil. δH (400 MHz; CDCl3; Me4Si) 0.89 (3H, t, J = 7.0 Hz, H-10), 1.28–1.36 (8H, m, H-6, H-7, H-8, H-9), 2.05 (2H, dt, J = 7.1 and 7.4 Hz, H-5), 2.48–2.53 (2H, m, H-2), 3.00 (3H, s, MsO), 4.21 (2H, t, J = 7.0 Hz, H-1), 5.30–5.37 (1H, m, H-3), 5.54–5.60 (1H, m, H-4). δC (100 MHz; CDCl3) 14.1 (C-10), 22.6 (C-9), 27.4, 27.4(C-2, C-5), 28.9 (C-7), 29.5 (C-6), 31.7, (C-8), 37.5 (MsO), 69.3 (C-1), 122.6 (C-3), 134.3 (C-4). IR: νmax(film)/cm–1; 2960, 2927, 2856, 1467, 1352, 1171, 953, 910, 803, and 734. MS data 70 eV, m/z (relative intensity) 234 (tr, M+), 138 (26), 109 (20), 95 (31), 81 (83), 68 (99), 67 (100), 55 (57), 54 (62) and 41 (45). HRMS (ESI+) found (MNa+): 257.1183 C11H22NaO3S requires 257.1182.
(Z)-Dec-3-enyl(octyl)sulfane (9)
Calcium ethoxide was prepared by adding CaH2 (546 mg, 13.0 mmol) to dry ethanol (5 mL). The resulting mixture was stirred overnight and then rigorously degassed using the freeze–pump–thaw22 technique and stored under argon. Octyl thiol (450 mg, 3.08 mmol) was weighed into a dry flask, and then mesylate (8) (760 mg, 3.24 mmol) was added along with 5 mL of dry ethanol. This mixture was also rigorously degassed using the freeze–pump–thaw22 technique, and the ethoxide solution prepared earlier was slowly added under argon. The reaction proceeded very slowly, and a second addition of thiol (450 mg, 3.08 mmol) was made after 7 d. The reaction was complete after 14 d, and the mixture was acidified with 1 M HCl. Water (25 mL) was added, and the organics were extracted with DCM (20 mL × 3). The combined organic extracts were then dried (MgSO4) and the solvent removed under vacuum. The crude thioether was then purified by column chromatography (silica gel, hexane) to give the title product (Z)-dec-3-enyl(octyl)sulfane (9) (495 mg, 1.74 mmol, 54% yield) as a clear colorless oil. δH (400 MHz; CDCl3; Me4Si 0.86–0.90 (6H, m, H-10, H-8′), 1.24–1.38 (18H, m, H-6, H-7, H-8, H-9, H-3′, H-4′, H-5′, H-6′, H-7′), 1.57–1.62 (2H, m, H-2′), 2.04 (2H, dt, J = 6.8 and 7.0 Hz, H-5), 2.33 (2H, dt, J = 7.2 and 7.4 Hz, H-2), 2.49–2.55 (4H, m, H-1, H-1′), 5.35–5.48 (2H, m, H-3, H-4). δC (100 MHz; CDCl3) 14.1 (C-10, C-8′), 22.7 (C-9, C-7′), 27.4 (C-5), 27.7 (C-2), 29.0, 29.2, 29.2, 29.6, 29.7 (C-6, C-7, C-2′, C-3′, C-4′, C-5′), 31.8, 31.8, (C-8, C-6′), 32.1, 32.2 (C-1, C-1′), 127.5 (C-3), 131.6 (C-4). IR: νmax(film)/cm–1; 2953, 2923, 2854, 1459, 1378, 967, and 723. MS data 70 eV, m/z (relative intensity) 284 (tr, M+), 255 (1), 171 (100), 159 (16), 129 (13), 87 (29), 69 (71), 61 (40), 55 (50) and 41 (37). HRMS (ESI+) found (MNa+): 307.2428 C18H36NaS requires 307.2430.
(Z)-1-(Octylsulfinyl)-dec-3-ene (10)
To a solution of thioether (9) (284 mg, 1.00 mmol), TeO2 (16 mg, 0.10 mmol), and HCl (cat.) in methanol (5 mL), 30% H2O2 (227 μL, 2.00 mmol) was added dropwise at room temperature with vigorous stirring. Upon completion of the reaction as shown by TLC, the organics were extracted with DCM (20 mL × 3) and then combined and washed with water (20 mL × 2) and dried (MgSO4). The solvent was removed under vacuum to give the title compound (Z)-1-(octylsulfinyl)-dec-3-ene (10) (262 mg, 0.87 mmol, 87% yield) as a clear colorless oil. The product was not purified further by column chromatography due to its instability and retention on the silica. Chemical purity was estimated at 92% based on NMR of the crude product. δH (400 MHz; CDCl3; Me4Si) 0.86–0.90 (6H, m, H-10, H-8′), 1.27–1.47 (18H, m, H-6, H-7, H-8, H-9, H-3′, H-4′, H-5′, H-6′, H-7′), 1.72–1.79 (2H, m, H-2′), 2.06 (2H, dt, J = 7.0 and 7.2 Hz, H-5), 2.53 (2H, dt, J = 7.8 and 8.2 Hz, H-2), 2.59–2.75 (4H, m, H-1, H-1′), 5.34–5.41 (1H, m, H-3), 5.480–5.51 (1H, m, H-4). δC (100 MHz; CDCl3) 14.1, 14.1 (C-10, C-8′), 20.7 (C-2), 22.6 (C-9, C-2′, C-7′), 27.3 (C-5), 28.9, 29.0, 29.0, 29.2, 29.5 (C-6, C-7, C-3′, C-4′, C-5′), 31.8 (C-8, C-6′), 52.3, 52.5 (C-1, C-1′), 125.6 (C-3), 133.0 (C-4). IR: νmax(film)/cm–1; 2955, 2924, 2855, 1459, 1409, 1377, 1026, and 723. MS data 70 eV, m/z (relative intensity): the product disintegrated in the GC–MS injection port. HRMS (ESI+) found (MNa+): 323.2375 C18H36NaOS requires 323.2379.
Insects
Mixed stage larval instars of raspberry bud moth were collected from wild blackberry plants in Chaney’s Forest (43°25′52.8″S 172°39′59.2″E) Christchurch, New Zealand. Larvae were reared through to pupation on fresh Rubus leaves inside an insect-proof mesh cage following a previously published method.23 Guava moth pupae were kindly gifted to us by Asha Chhagan. Individual pupae were maintained at 20–24 °C in humidified 30 mL plastic containers until emergence.
EAG Recording
This methodology has been previously reported in detail, so is only briefly described here.11 Antennal depolarizations from 1–6 day old virgin males of both moth species were recorded on an IDAC-4 (Okenfels Syntech, GmbH, Kirchzarten, Germany) to the synthetic compounds. Antennal preparations consisted of an excised antenna mounted between silver electrodes housed in saline, standing in a humidified air stream (600 mL/min). The individual synthetic compounds were presented to the antenna in the form of a single puff from premade odor cartridges upwind in the air stream. Odor cartridges consisted of a 1 cm2 piece of filter paper (Whatman no.1, USA) containing the desired amount of compound housed in a glass Pasteur pipette. Based on previous EAG testing of carposinid moths by Twidle et al., where moths were sensitive to pheromone loadings in the 100 ng to 1 μg range,11 a dose of 10 μg was chosen for the antennal testing. For all tetrahedral analogues, which had been prepared as a racemic mixture, a dose of 20 μg was used to ensure each enantiomer was present in a 10 μg loading. The maximum antennal depolarization in response to the puff was recorded using the IDAC-4 coupled with Syntech EAD Pro software. Statistical calculations were made using Minitab 18 software. The homogeneity of variance of the antennal depolarization data was checked, and the data for each moth species were transformed by log10 (μV + 1) to stabilize variance. Separate statistical analyses were run for each species of moth. The transformed data were analyzed by one-way ANOVA, and the treatments were compared using Tukey’s pairwise comparisons (P < 0.05).
GC-EAD
Antennal preparations for both moth species were prepared as described above. These were then placed in a charcoal filtered, humidified airstream adjacent to the Agilent 7890B GC effluent port. The effluent port of the gas chromatograph entered the main airstream approximately 10 cm up wind from the antennal preparation. One μL of concentrated alcohol analogue solution was then injected into the gas chromatograph. The injector of the gas chromatograph was set at 250 °C, and the injection was splitless for 0.6 min. The gas chromatograph oven ramp consisted of a 2 min hold at 40 °C and then increased by 10 °C/min up to 280 °C. The gas chromatograph oven was equipped with a DB-5ms column (30 m × 0.25 mm i.d. × 0.25 μm) and used helium as the carrier gas with a flow rate of 1.2 mL/min. A Syntech IDAC-4 recording unit and Syntech GC–EAD software recorded maximum antennal depolarizations from the antennal preparation, along with peak areas and retention times from the gas chromatograph. The alcohol analogue solution was tested on the male antennae of three individuals from each moth species.
Acknowledgments
We thank Rayonier Inc. for allowing us access to Chaney’s Forest for raspberry bud moth collection and Asha Chhagan for providing us with guava moth for antennal testing. We also thank Lee-Anne Manning and Barry Bunn for constructive comments on the manuscript and Rikard Unelius for insightful discussions regarding analogue structures.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c02614.
NMR spectra of synthesized compounds (PDF)
Funding was provided by The New Zealand Ministry for Primary Industries (MPI-SFF), New Zealand Feijoa Growers Association, New Zealand Macadamia Society, Northland Regional Council, UPL, Hawke’s Bay Regional Council, and the Gisborne District Council.
The authors declare no competing financial interest.
Supplementary Material
References
- Heppner J. B.Fruitworm moths (Lepidoptera: Carposinidae). In Encyclopedia of Entomology; Capinera J. L., Ed.; Springer: Dordrecht, Netherlands, 2008; p 1541. [Google Scholar]
- Jamieson L. E.; Dymock J. J.; Dawson T. E.; Froud K. J.; Seldon D. S.; Suckling D. M.; Gibb A. R. Guava moth in New Zealand distribution hosts life cycle observations and discussion of pesticide management options. N. Z. Plant Prot. 2004, 57, 13–19. 10.30843/nzpp.2004.57.6934. [DOI] [Google Scholar]
- Tamaki Y.; Honma K.; Kawasaki K. Sex pheromone of the peach fruit moth, Carposina niponensis Walsingham (Lepidoptera : Carposinidae) : Isolation, identification and synthesis. Appl. Entomol. Zool. 1977, 12, 60–68. 10.1303/aez.12.60. [DOI] [Google Scholar]
- Foster S. P.; Thomas W. P. Identification of a sex pheromone component of the raspberry budmoth, Heterocrossa rubophaga. J. Chem. Ecol. 2000, 26, 2549–2555. 10.1023/a:1005584628955. [DOI] [Google Scholar]
- Gibb A. R.; Suckling D. M.; Morris B. D.; Dawson T. E.; Bunn B.; Comeskey D.; Dymock J. J. (Z)-7-Tricosene and monounsaturated ketones as sex pheromone components of the Australian guava moth Coscinoptycha improbana: Identification, field trapping, and phenology. J. Chem. Ecol. 2006, 32, 221–237. 10.1007/s10886-006-9361-z. [DOI] [PubMed] [Google Scholar]
- Twidle A. M.; Barker D.; Suckling D. M. (7Z)-Tricosene improves pheromone trap catch of raspberry bud moth, Heterocrossa rubophaga. J. Chem. Ecol. 2020, 46, 830–834. 10.1007/s10886-020-01205-2. [DOI] [PubMed] [Google Scholar]
- Ando T.; Inomata S.; Yamamoto M.. Lepidopteran sex pheromones. In The Chemistry of Pheromones and Other Semiochemicals I; Schulz S., Ed.; Springer Heidelberg: Germany, 2004; pp 51–96. [DOI] [PubMed] [Google Scholar]
- van der Geest L. P. S.; Evenhuis H. H.. Tortricid Pests: Their Biology. Natural Enemies and Control; Elsevier Science: Amsterdam, The Netherlands, 1991; Vol. 5, pp 808. [Google Scholar]
- Renou M.; Guerrero A. Insect parapheromones in olfaction research and semiochemical-based pest control strategies. Annu. Rev. Entomol. 2000, 45, 605–630. 10.1146/annurev.ento.45.1.605. [DOI] [PubMed] [Google Scholar]
- Suckling D. M.; Dymock J. J.; Park K. C.; Wakelin R. H.; Jamieson L. E. Communication disruption of guava moth (Coscinoptycha improbana) using a pheromone analog based on chain length. J. Chem. Ecol. 2013, 39, 1161–1168. 10.1007/s10886-013-0339-3. [DOI] [PubMed] [Google Scholar]
- Twidle A. M.; Suckling D. M.; Chhagan A.; Pilkington L. I.; Park K. C.; Barker D. Synthesis and biological testing of ester pheromone analogues for two fruitworm moths (Carposinidae). J. Agric. Food Chem. 2020, 68, 9557–9567. 10.1021/acs.jafc.0c04131. [DOI] [PubMed] [Google Scholar]
- Sellanes C.; Rossini C.; González A. Formate analogs as antagonists of the sex pheromone of the honeydew moth, Cryptoblabes gnidiella: Electrophysiological, behavioral and field evidence. J. Chem. Ecol. 2010, 36, 1234–1240. 10.1007/s10886-010-9861-8. [DOI] [PubMed] [Google Scholar]
- Camps F.; Gasol V.; Guerrero A. Inhibitory pheromonal activity promoted by sulfur analogs of the sex pheromone of the female processionary moth Thaumetopoea pityocampa (Denis and schiff). J. Chem. Ecol. 1990, 16, 1155–1172. 10.1007/bf01021017. [DOI] [PubMed] [Google Scholar]
- Coumbarides G. S.; Eames J.; Weerasooriya N. A practical laboratory route to the synthesis of trideuteriomethyl-[13C] iodide. J. Labelled Compd. Radiopharm. 2003, 46, 291–296. 10.1002/jlcr.666. [DOI] [Google Scholar]
- Kwan S. K.; Hye J. H.; Chan S. C.; Chi S. H. Tellurium dioxide catalyzed selective oxidation of sulfides to sulfoxides with hydrogen peroxide. Tetrahedron Lett. 1990, 31, 2893–2894. 10.1016/0040-4039(90)80176-m. [DOI] [Google Scholar]
- Bäckman A.-C.; Anderson P.; Bengtsson M.; Löfqvist J.; Unelius C. R.; Witzgall P. Antennal response of codling moth males, Cydia pomonella L. (Lepidoptera: Tortricidae), to the geometric isomers of codlemone and codlemone acetate. J. Comp. Physiol., A 2000, 186, 513–519. 10.1007/s003590000101. [DOI] [PubMed] [Google Scholar]
- Lucas P.; Renou M.; Tellier F.; Hammoud A.; Audemard H.; Descoins C. Electrophysiological and field activity of halogenated analogs of (E,E)-8,10-dodecadien-1-ol, the main pheromone component, in codling moth (Cydia pomonella L.). J. Chem. Ecol. 1994, 20, 489–503. 10.1007/bf02059592. [DOI] [PubMed] [Google Scholar]
- Ding Y.-S.; Prestwich G. D. Chemical studies of proteins that degrade pheromones. J. Chem. Ecol. 1988, 14, 2033–2046. 10.1007/bf01014248. [DOI] [PubMed] [Google Scholar]
- El-Sayed A. M.The Pherobase: Database of Insect Pheromones and Semiochemicals. http://www.pherobase.com. 2003–2021-The Pherobase - Ashraf M. El-Sayed, 2021.
- Twidle A. M.; Mas F.; Harper A. R.; Horner R. M.; Welsh T. J.; Suckling D. M. Kiwifruit flower odor perception and recognition by honey bees, Apis mellifera. J. Agric. Food Chem. 2015, 63, 5597–5602. 10.1021/acs.jafc.5b01165. [DOI] [PubMed] [Google Scholar]
- Alonso F.; Osante I.; Yus M. Highly selective hydrogenation of multiple carbon-carbon bonds promoted by nickel(0) nanoparticles. Tetrahedron 2007, 63, 93–102. 10.1016/j.tet.2006.10.043. [DOI] [Google Scholar]
- Perrin D. D.; Armarego W. L. F.. Purification of laboratory chemicals, 3rd ed.; Pergamon Press: Australia, 1988; p 391. [Google Scholar]
- Clare G. K.; Singh P. Laboratory rearing of the raspberry bud moth, Heterocrossa rubophaga (Lepidoptera: Carposinidae) on blackberry. N. Z. J. Zool. 1991, 18, 89–91. 10.1080/03014223.1991.10757952. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.