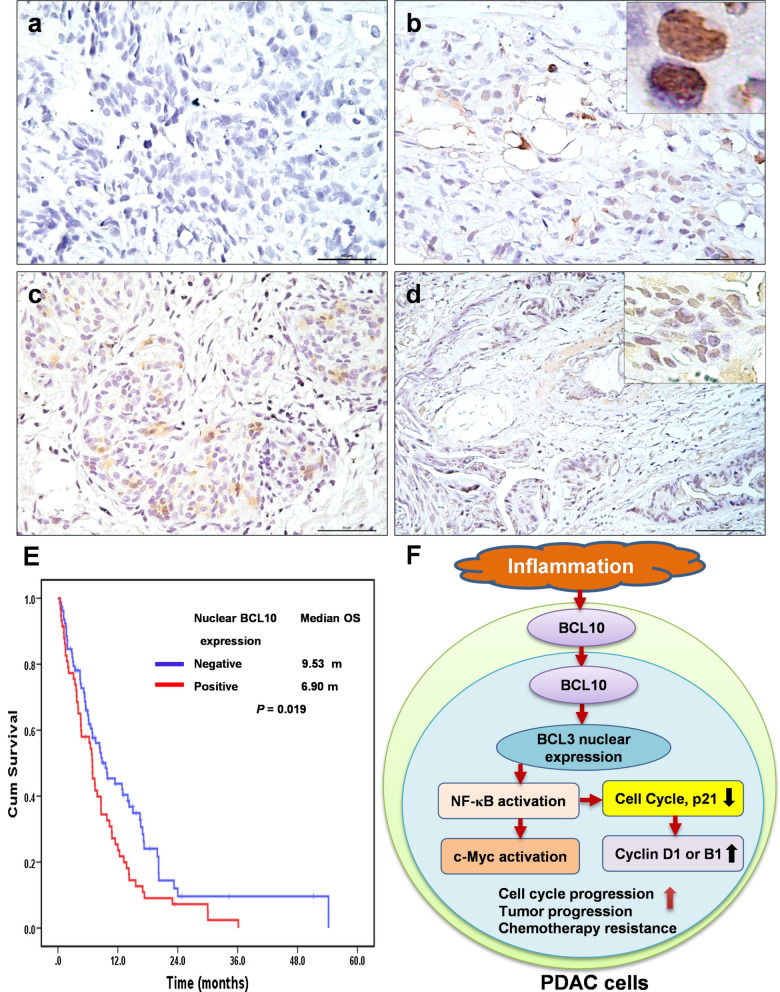
Fig. 5.
Expression of nuclear BCL10 in tumor cells and its association with survival of patients with recurrent, advanced and metastatic PDAC. a Representative images of negative BCL10 expression in tumor specimens of PDAC. b Representative images of nuclear BCL10 expression in biopsy tumor specimens of PDAC. Right upper inset (nuclear expression of BCL10, 100X magnification). c Representative images of moderate nuclear BCL10 expression in tumor specimens of PDAC. d Representative images of strong nuclear BCL10 expression in tumor specimens of PDAC. Right upper inset (nuclear expression of BCL10, 40X magnification). e The Kaplan–Meier overall survival (OS) for all patients associated with the expression of nuclear BCL10. f Inflammation-related signals in the tumor microenvironment of PDAC upregulates BCL10 expression and induces BCL10 nuclear translocation; BCL10 nuclear expression results in BCL3 nuclear translocation and the activation of NF-κB. The activation of NF-κB further triggers its regulating transcriptional factors, including c-Myc and cell cycle proteins, such as Cyclin D1 in wild-type KRAS PDAC, and Cyclin B1 in KRAS mutant PDAC. These signaling pathways further promote cell cycle progression, tumor progression, and contribute to chemotherapy resistance of PDAC. Inhibition of nuclear BCL10 translocation attenuates the activation of NF-κB, leading to the decreased expression of its regulatory cell cycle proteins, Cyclin D1 (wild-type KRAS) or Cyclin B1 (mutant KRAS), and the altered expression of p21, and thus fosters G1 arrest and G2/M arrest, respectively, and also enhances chemosensitivity and prolongs the survival of patients with PDAC. PDAC pancreatic ductal adenocarcinoma