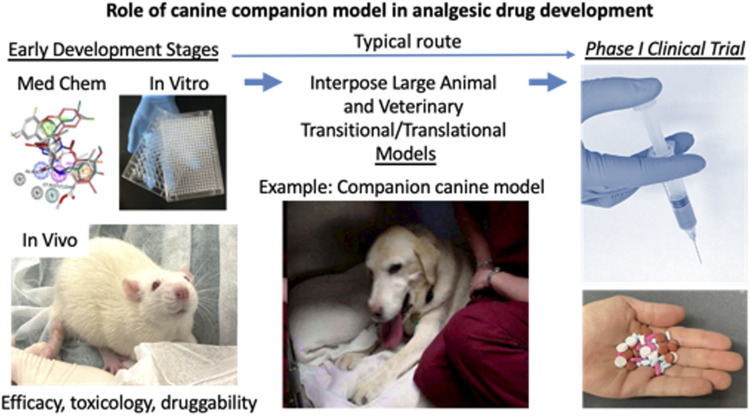
FIGURE 1.
Role of companion canine model in analgesic drug development. The drug development process progresses from left to right. The early development steps include, discovery, medicinal chemistry, in vitro screening, in vivo testing in rodent models, and optimization for target engagement, adsorption, distribution, metabolism, excretion. Usually, after manufacturing processes and toxicology are conducted, the candidate molecule goes into phase I testing. What is often lacking, despite positive result from mouse or rat studies is a clear indication of whether the compound produces analgesia in clinical pain states. These would be indications arising from natural diseases. To address this gap the veterinary companion canine model is interposed between the two major milestones in drug development. The two main clinical conditions that have been used for analgesic drug evaluation are pain from osteosarcoma and osteoarthritis. These conditions both impact motor activity and performance which provide obvious endpoints for evaluation. Since the pain from the conditions affects activities of daily living, the owners have multiple parameters from which to judge improvement in their animal’s behavior. Formal, validated scales have been developed for evaluation of both pain severity and pain interference with activity (Brown et al., 2007; Brown et al., 2008; Brown et al., 2009; Brown et al., 2010; Brown et al., 2013a; Brown et al., 2013b; Brown, 2014). Other parameters available include objective measures such as force plate determinations of weigh bearing and analysis of video recordings. Because a weight bearing limb (or limbs) is involved in both disorders and either a fore or hind limb can be affected, gait and force plate measurements sometimes can be difficult to obtain in a reproducible or reliable manner (Iadarola et al., 2018). However, the range of potential available endpoints is broad enough to be adapted to a variety of conditions.