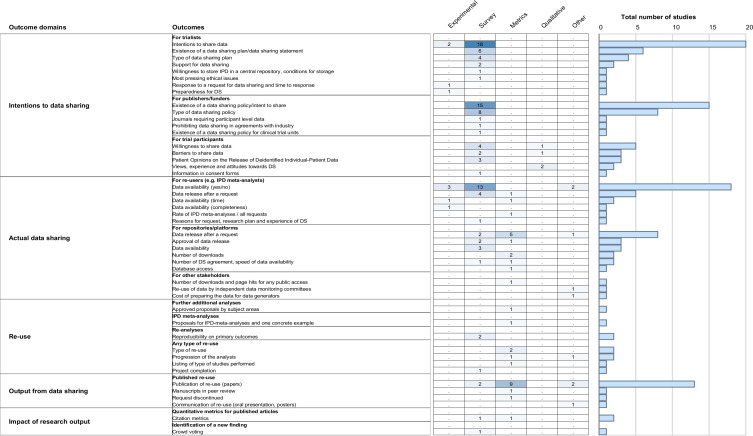
Figure 3.
Outcomes used to assess current data-sharing practices for individual patient data for clinical trials organised per outcome domain and number of studies exploring these outcomes. Study designs considered. Experimental: prospective research that implies testing the impact a strategy (eg, randomised controlled trial). Survey: a general view, exploration or description of individuals and/or research objects. Metrics: descriptive metrics from each initiative provided by the initiative. Qualitative: research that relies on non-numerical data to understand concepts, opinions or experiences. Other: any other research not covered above (eg, case studies, environmental scans). IPD, individual participant data.