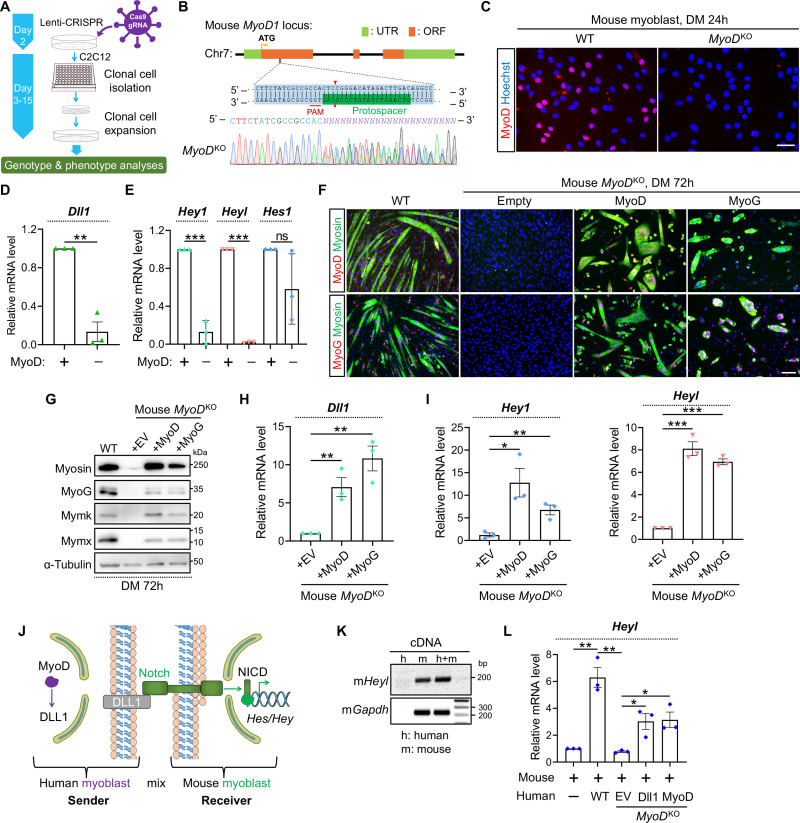
Fig 6. MyoD is essential for Dll1 expression in mouse myoblasts.
(A) Schematic of CRISPR/Cas9 approach to generate gene-knockout clones from mouse C2C12 myoblasts. (B) Sanger sequencing results of mouse MyoD genotyping PCR products. (C) Myod immunostaining confirmed depletions of MyoD protein in MyoDKO clones. Scale bar, 50 μm. (D, E) qPCR results of Dll1 (D) and Notch target genes (E) in WT and MyoDKO myoblasts. Cells were differentiated for 48 hours. n = 3. (F) Immunostaining results of MyoD (top) or MyoG (bottom) together with myosin. Note that MyoDKO myoblasts failed to differentiate and such a defect was rescued by retroviral expression of MyoD or MyoG. Cells were differentiated for 72 hours. Scale bar, 100 μm. (G) Western blotting results to show the expression levels of muscle proteins in WT or MyoDKO myoblasts. Cells were differentiated for 72 hours. (H, I) qPCR results of Dll1 (H) and Notch target genes (I) in mouse MyoDKO cells. Myoblasts were differentiated for 72 hours. n = 3. (J) Schematic of Notch signaling that depicted the rationale of human and mouse myoblast-mixing assays. (K) Gel electrophoresis result that validated the specificity of mouse qPCR primers. (L) qPCR results that measured mRNA level of mouse Heyl using primers validated in K. Before mixing with mouse myoblasts, WT or MyoDKO Human myoblasts were infected by retroviruses expressing Dll1 or MyoD. Cells were differentiated for 24 hours. n = 3. Data are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. ns, not significant.