Figure 4 – Hematopoietic differentiation of the DKC1[A353V] iPSCs.
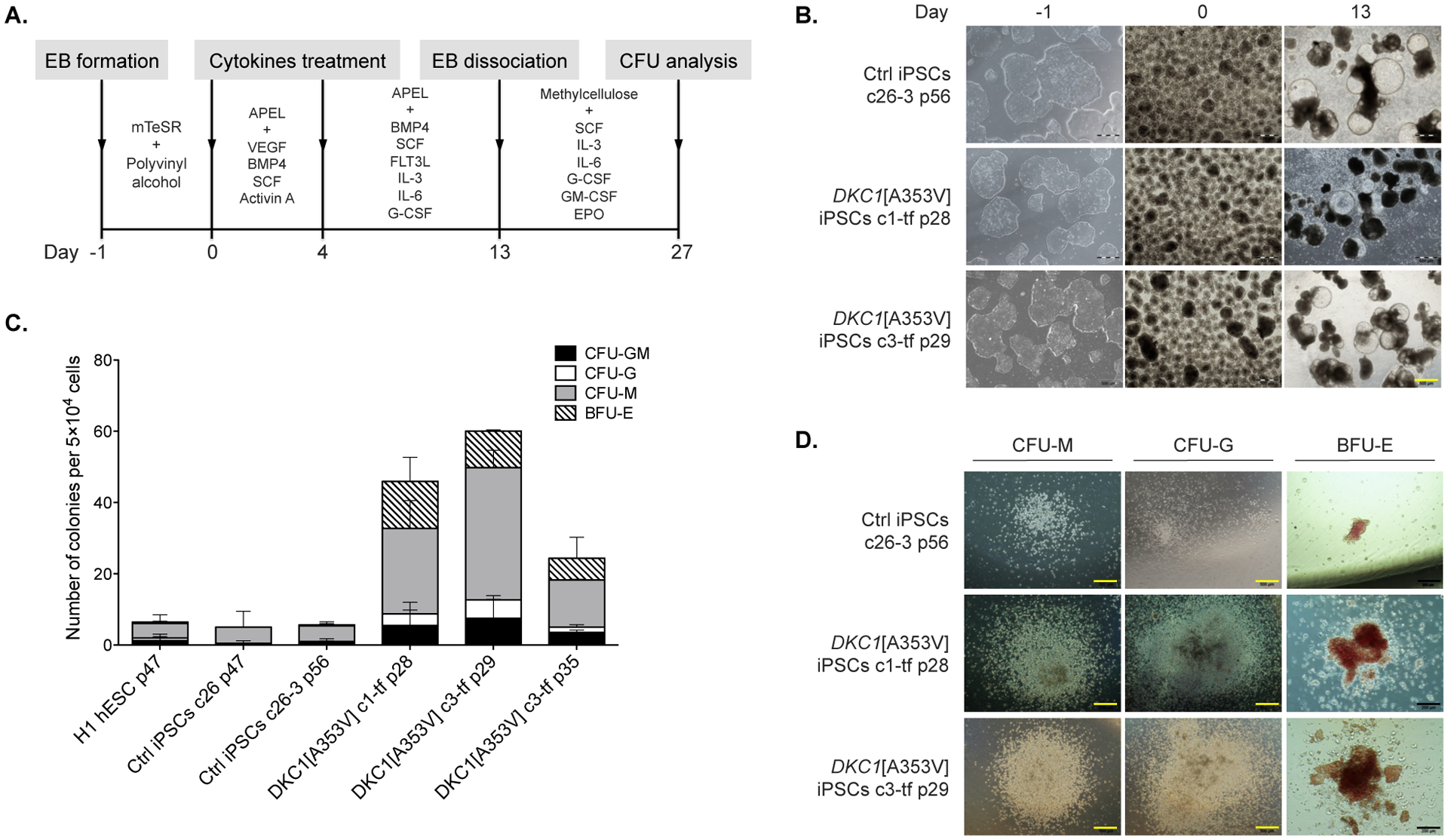
(A) Overview of differentiation: embryoid bodies (EBs) were derived from the iPSCs and guided to mesodermal differentiation with specific cytokines for three days (day 0 to 3). By day 4, the medium was replaced with a different cocktail of cytokines that promote maturation of the hematopoietic stem and progenitor cells. On day 13, EBs were harvested and dissociated, and single cells were seeded in methylcellulose containing cytokines. CFUs formed after 14 days in methylcellulose were classified and counted. (B) The iPSCs (control and DKC1-mutants) are shown during the differentiation process, from iPSC colonies in the undifferentiated state (on day −1), EBs on day 0, to differentiated EBs with cystic structures on day 13. Both iPSC mutants and control exhibited no morphological differences that could be detected under the inverted microscope during the differentiation. Yellow scale bar = 500 μm. (C) Qualitative and quantitative analysis of hematopoietic colonies derived from controls (H1 hESC, c26 and c26-3 iPSCs) as well as clones (c) of DKC1-mutant iPSCs at distinct passages (p). After CFU analysis, the DKC1-mutant iPSCs were found to be more prone to differentiate into myeloid lineages (c1-tf p28 and c3-tf p29). Counts are represented as the average and standard error of three independent experiments. CFU-GM, CFU-granulocyte, macrophage; CFU-G, CFU-granulocyte; CFU-M, CFU-macrophage; BFU-E, burst forming unit-erythroid. (D) Morphology of CFUs generated from iPSC control and DKC1-mutant clones. Black scale bars = 200 μm.
