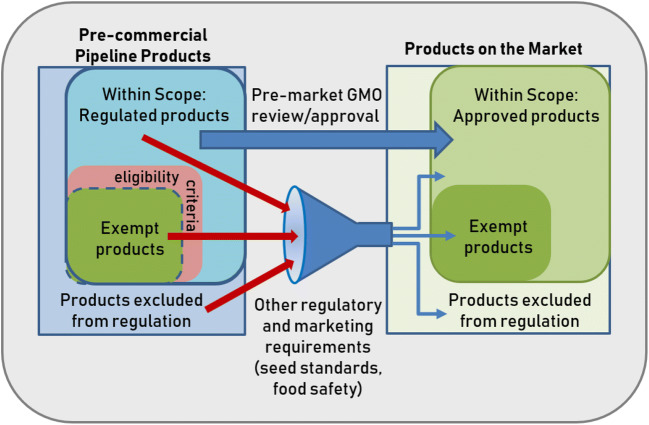
Figure 1.
Schematic diagram illustrating the general relationship between regulatory scope, exclusions, and exemptions, and the role of eligibility criteria for plant products. Products that are, by definition, within the scope of the regulations may either be evaluated by a regulatory body and approved to gain market access or determined to meet defined exemption criteria (“eligibility criteria”) and considered to meet the same safety or protection targets as products that have been approved. It should be noted that all products whether they are “excluded from regulation,” exempted or go through a pre-market approval process would be subject to other regulatory requirements under other authorities (e.g., seed testing/quality standards, food safety standards). Depending on the country/region, a myriad of post-market safety and marketing requirements are also likely to be applicable to all products (Institute of Medicine (US) and National Research Council (US) 1998).