Scheme 3. Hypothetical location and function of Y385 NS1c tyrosyl radical.
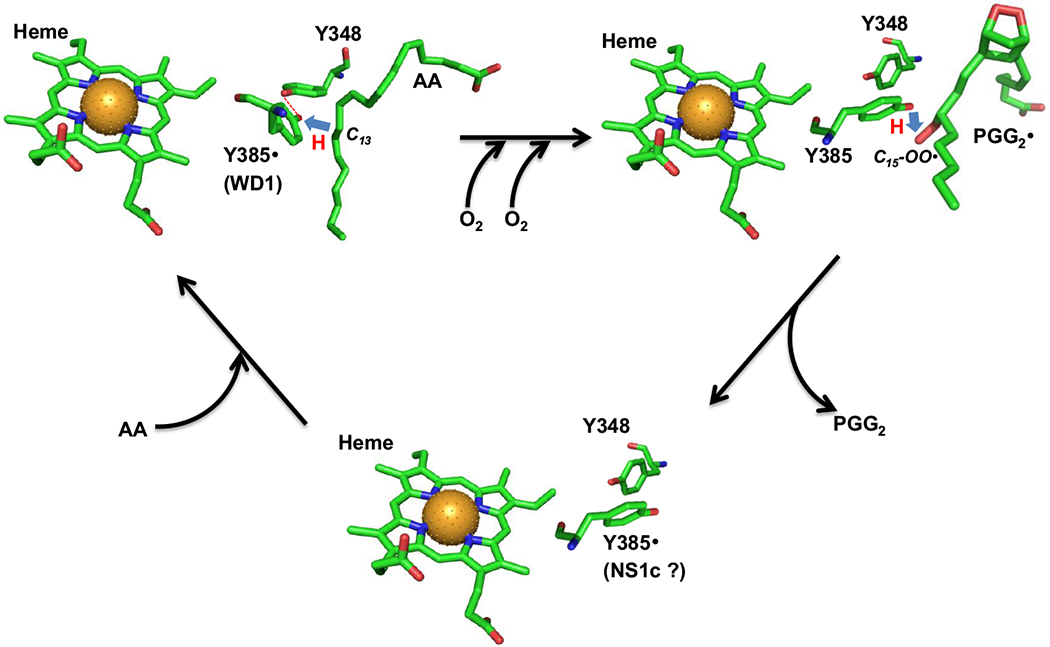
In AA-bound Intermediate II, Y385· is H-bonded to Y348 (red dashed line) and exhibits a wide doublet EPR lineshape (WD1 radical). Y385· abstracts hydrogen from AA C13 atom (blue arrow) to generate arachidonyl carbon-centered radical. The arachidonyl radical is then converted to PGG2· radical through a series of COX reactions including insertion of two oxygen molecules, ring formations and radical shifts (horizontal black arrow). After generation of the arachidonyl radical, the H-bond between Y348 and Y385 is disrupted and Y385 adopts a new conformation ready for hydrogen transfer back from Y385 to C15-OO· of PGG2· radical (blue arrow) to generate PGG2 and regenerate Y385· radical. After the release of PGG2 and binding of the next AA molecule (tilted black arrows), H-bond between Y348 and Y385· radical is re-established. The AA-bound PGHS-1 structure is based on crystal structure 1DIY (PDB code) for AA-bound PGHS-1 with Co protoporphyrin IV. The different conformers of Y385 and Y348 in the other two structures are arbitrarily plotted and the chemical structure of PGG2 is based on the calculated structure 1DD0 (PDB code). All the structures are plotted using PyMOL. Green: carbon; red: oxygen; blue: nitrogen and brown ball: cobalt atom. (The color version of the figure is available in the electronic copy of the article).
