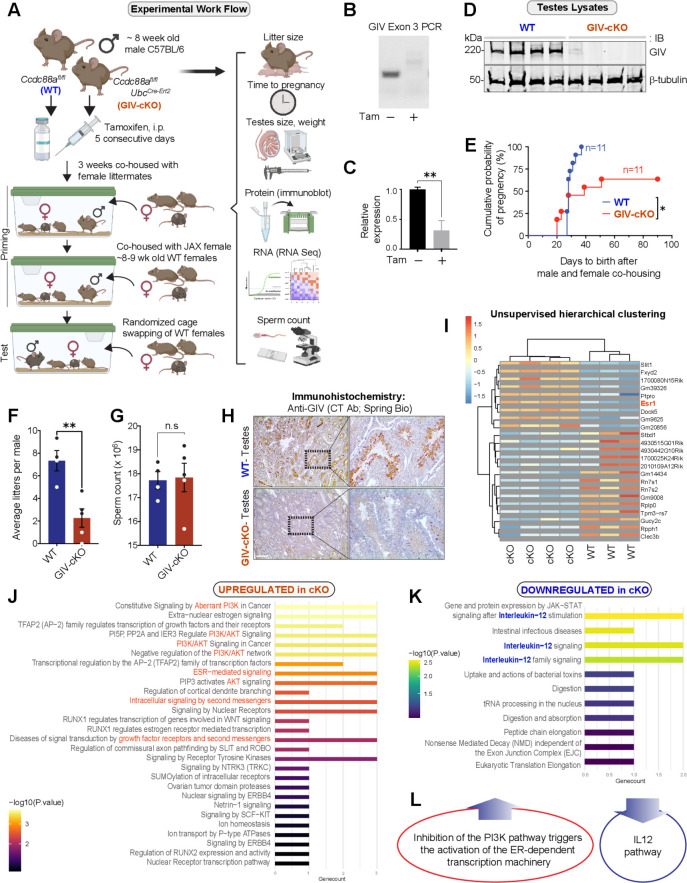
Figure 5. GIV is required for fertility in male mice.
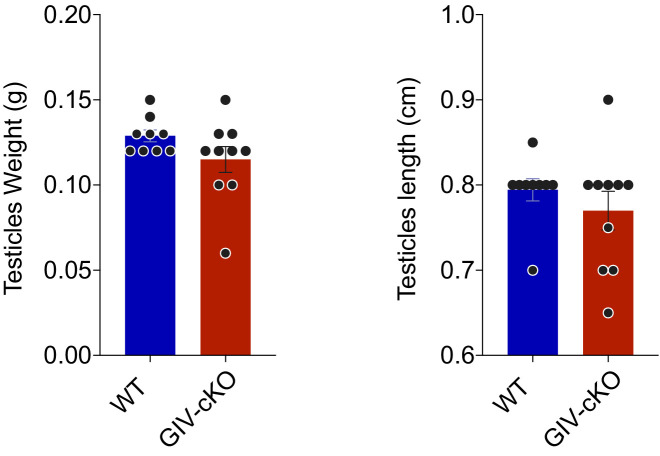
(A) Schematic showing the workflow for fertility studies in conditional GIV-cKO mice. After intraperitoneal injection of tamoxifen, male mice were first primed in two phases—first by co-housing with female littermates × 3 weeks, and subsequently by co-housing with female mice from Jackson laboratory (JAX) while the females acclimatized to the animal facility. The final ‘test’ group consisted of tamoxifen-injected WT and GIV-cKO male mice randomly assigned to and co-housed with three female mice from JAX, each with proven ability to get pregnant. (B–D) Confirmation of GIV-cKO in the mice after tamoxifen injection by genotyping (B), qPCR of testis tissues (C), and immunoblotting of testis lysates (D) (Figure 5—source data 1). (E) Kaplan–Meier plot showing the cumulative probability of pregnancy (expressed as %) in the females co-housed with either WT or GIV-cKO males. Statistical significance was assessed using log-rank analysis. *p<0.05 (Figure 5—source data 2). (F, G) Bar graphs showing the average litter size (F; Figure 5—source data 3) and sperm count (G; Figure 5—source data 4) in WT and GIV-cKO males. See also Figure 5—figure supplement 1 for quantifications of tested weight and length. (H) Immunohistochemistry staining on mouse testis. Scale bar = 200 µm. (I) Unsupervised clustering of WT and KO testis samples based on gene expression. Differentially expressed genes (DEGs) that were up- or downregulated in KO are annotated on the right side (Figure 5—source data 5). (J, K) Reactome pathway analyses showing the pathways that are up or downregulated in KO testis. (L) Summary of the most prominent conclusions from RNA-seq dataset.