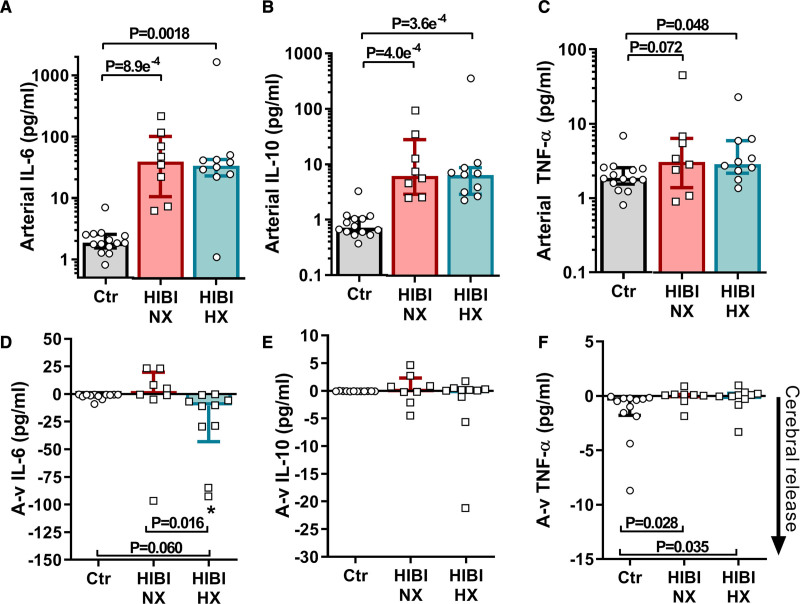
Figure 5.
Cerebral inflammation is associated with secondary brain hypoxia. Arterial and jugular venous blood draws were acquired for both healthy controls (Ctr) and for patients with hypoxic ischemic brain injury (HIBI) with brain normoxia (NX; red) and hypoxia (HX; cyan). Data are presented as bars (median±IQR) with individual data overlaid for each group. Arterial serum biomarker concentrations for IL-6 (interleukin-6; A), interleukin-10 (IL-10; B) and TNF-α (tumor necrosis factor alpha; C) are presented on the top row. For arterial serum IL-6 and IL-10, both HIBI groups had elevated levels compared with controls, while only patients with HIBI with brain hypoxia had higher arterial TNF-α levels than controls. Simultaneous collection of arterial and jugular venous blood, and calculation of the consequent arterial-to-venous gradient (A-v) allows for determination of biomarker uptake/release from the brain, where a negative (−) A-v gradient indicates biomarker release from the brain. These data for each biomarker are depicted on the second row in D–F. The cerebral release for IL-6 in the normoxic HIBI group was not different from that of controls, whereas the patients with hypoxic HIBI had a greater cerebral release than controls and patients with normoxic HIBI. The cerebral release of IL-10 did not differ between any group while there was a trivial difference between the TNF-α A-v gradient in the control group and both HIBI groups. Significance testing between groups was conducted with a Kruskal-Wallis test. Following the detection of a significant effect, post hoc testing was conducted and corrected for multiple comparisons with a Dunn’s multiple comparisons test. An asterisk (*) indicates that an A-v gradient (cerebral release) is significantly different from zero. P<0.05 was considered statistically significant.