Figure 2.
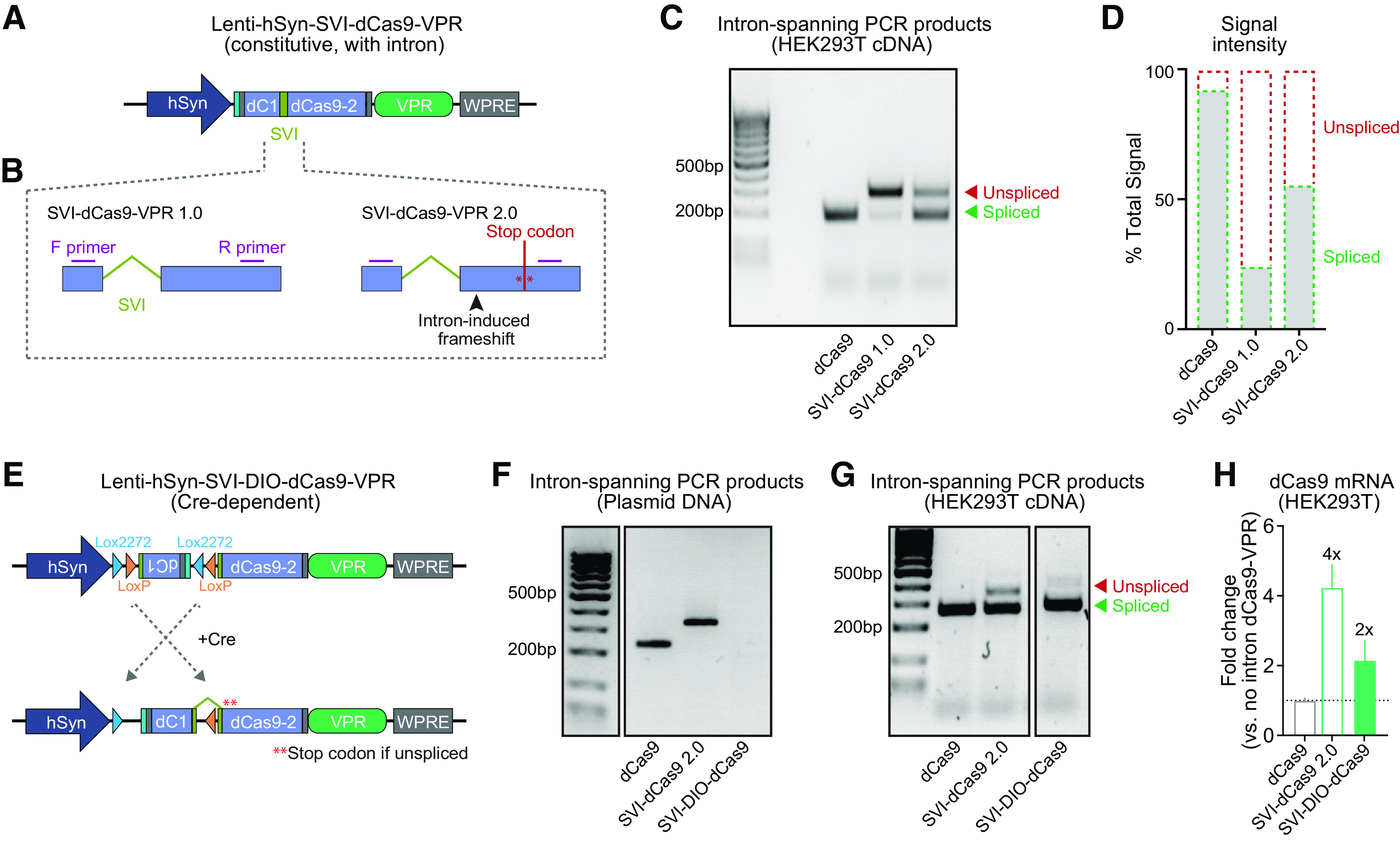
Design of an intron-containing, Cre-dependent CRISPRa system. A, Illustration of CRISPR construct design for an intron-containing constitutive dCas9-VPR cassette. B, Design of the two different intron positions. C, SVI-dCas9-VPR 1.0 and 2.0 intron positions were tested in HEK293T cells. PCR amplification of cDNA with primers spanning the intron within dCas9 showed more efficient splicing of the SVI-dCas9-VPR 2.0 construct. D, Quantification of PCR product intensities shown in panel C. E, Illustration of a Cre-dependent SVI-DIO-dCas9-VPR construct in which the first dCas9 segment is double-floxed and inverted. F, PCR amplification of plasmid DNA with intron-spanning primers verify that no product results from the SVI-DIO-dCas9-VPR plasmid before recombination. G, PCR amplification of cDNA following HEK293T cell transfection with dCas9-VPR, SVI-dCas9-VPR, and SVI-DIO-dCas9-VPR with Cre showed that SVI-DIO-dCas9-VPR transfected cells express spliced dCas9-VPR transcripts in the presence of Cre recombinase. H, Intron insertion increases dCas9-VPR mRNA expression, detected with RT-qPCR for a common region within dCas9 (n = 8 per group). Kruskal–Wallis test F(3,24) = 14.62, p = 0.0007; Multiple comparisons tests p = 0.0003 for SVI-dCas9 2.0 versus constitutive dCas9, and p = 0.0357 for SVI-DIO-dCas9 (with Cre recombinase) versus constitutive dCas9. Data expressed as mean ± SEM.
