FIGURE 1.
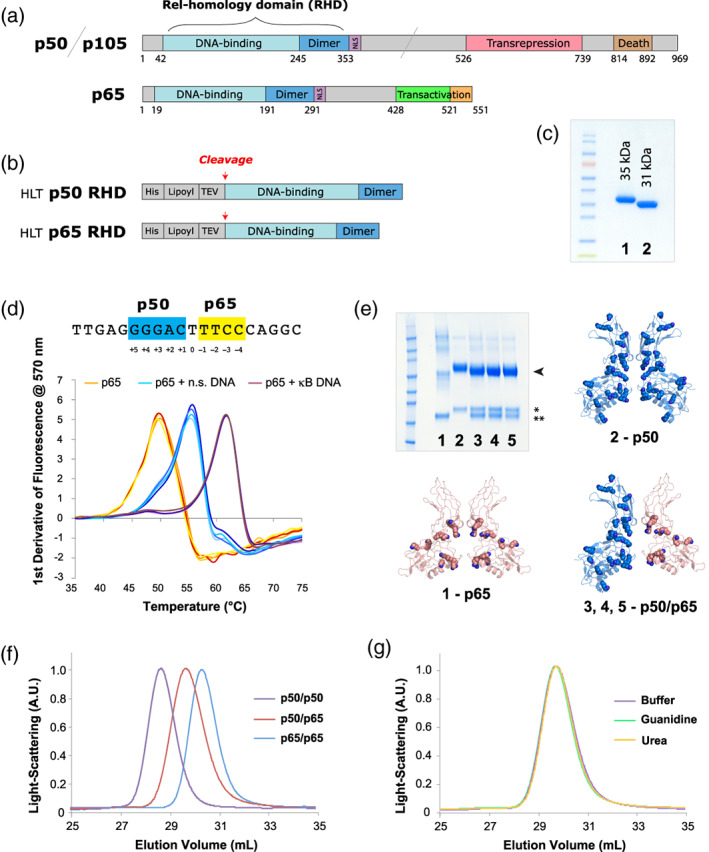
Recombinant proteins used in this work. (a) The full‐length variants of p50/p105 and p65. (b) The HLT fusion proteins used for expression and purification. (c) SDS‐PAGE gel of purified samples. (All samples used in this figure were the first‐generation constructs, for example, p50‐1 RHD and p65‐1 RHD, described here briefly as p50 RHD and p65 RHD.): 1–p50 RHD, 2–p65 RHD. (d) Nucleotide binding by NF‐κB. The sequence of the canonical p50/p65 heterodimer Ig‐like binding site is shown with the p50 half‐site in blue and the p65 half‐site in yellow. Below are shown DSF temperature melts of the p65 RHD subunits of NF‐κB (with each condition showing four repeats, in slight variations of the colors listed below). p65 RHD was heat‐denatured in 50 mM NaCl buffer in the absence of oligonucleotides (orange), presence of heparin or nonspecific DNA (cyan), and presence of specific κB site DNA (purple). Four repeats of each melt (varying shades of the colors above) are shown. (e) SDS‐PAGE of crosslinked samples of various RHD preparations, stained with Coomassie blue: 1–p65 RHD, 2–p50 RHD, 3–p50/p65 RHD mixed in buffer, 4–p50/p65 RHD refolded from urea denaturation, 5–p50/p65 RHD refolded from GuHCl denaturation. The bands corresponding to dimeric crosslinked species are indicated with an arrowhead, and the isolated p50 (*) and p65 (**) monomers are indicated with asterisks. The structure of the p50 (blue, PDB ID: 1SVC) and p65 (peach, PDB ID: 2RAM) homodimers, as well as the p50/p65 heterodimer (blue/peach, PDB ID: 1VKX)—all of which have had their corresponding double‐stranded DNA oligos omitted—are shown as cartoon representations, with lysine side chains depicted as spheres. (f) SEC analysis of p50 RHD (purple) and p65 RHD (blue) homodimers, as well as the p50/p65 RHD heterodimer (red). (g) Gel filtration of the heterodimer species generated either by directly mixing the p50 and p65 RHD homodimers (purple), or by denaturing the RHD homodimers in GuHCl (green) or urea (orange), followed by refolding via overnight dialysis into buffer. DSF, differential scanning fluorimetry; GuHCl, guanidinium hydrochloride; HLT, His‐lipoyl‐TEV; RHD, Rel homology domain; SDS‐PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; SEC, size‐exclusion chromatography
