
Key words: Quarantine, Lockdown, Body weight, Obesity, Weight determinants, Pandemic
Pandemics and subsequent lifestyle restrictions such as ‘lockdowns’ may have unintended consequences, including alterations in body weight. This systematic review assesses the impact of pandemic confinement on body weight and identifies contributory factors. A comprehensive literature search was performed in seven electronic databases and in grey sources from their inception until 1 July 2020 with an update in PubMed and Scopus on 1 February 2021. In total, 2361 unique records were retrieved, of which forty-one studies were identified eligible: one case–control study, fourteen cohort and twenty-six cross-sectional studies (469, 362 total participants). The participants ranged in age from 6 to 86 years. The proportion of female participants ranged from 37 % to 100 %. Pandemic confinements were associated with weight gain in 7·2–72·4 % of participants and weight loss in 11·1–32·0 % of participants. Weight gain ranged from 0·6 (sd 1·3) to 3·0 (sd 2·4) kg, and weight loss ranged from 2·0 (sd 1·4) to 2·9 (sd 1·5) kg. Weight gain occurred predominantly in participants who were already overweight or obese. Associated factors included increased consumption of unhealthy food with changes in physical activity and altered sleep patterns. Weight loss during the pandemic was observed in individuals with previous low weight, and those who ate less and were more physically active before lockdown. Maintaining a stable weight was more difficult in populations with reduced income, particularly in individuals with lower educational attainment. The findings of this systematic review highlight the short-term effects of pandemic confinements.
Devastating physical morbidity and mortality outcomes due to coronavirus disease 2019 (COVID-19) have been mitigated by(1,2) social distancing and quarantine measures(3), with significant direct and indirect health implications. Although lockdown has reduced the ‘R number’, physical well-being may have suffered from increased levels of stress, anxiety and mental health issues(4–6). Moderate weight gain in people with a normal BMI has an adverse effect on metabolism, which increases the risk of diabetes, CVD(7) or long-term ill-health(8). Lockdown may precipitate weight gain similar to that seen during the 6-week summer holidays because of increased inactive time spent at home and snacking on energy-dense foods(9–11). Rundle and colleagues argued that the extent and haste of the restrictions have exaggerated these observations(12) leading to rapid weight gain. This presents particular issues with the gained weight being more difficult to shed(13). Moreover, physical and social isolation is a recognised risk factor for obesity(14), with weight due to overconsumption, particularly when large “emergency” food stores are present(15). Reduced physical activity has further exacerbated the weight gain.
The COVID-19 outbreak adversely affected food supply and demand on a global scale(16). For some, lockdown gave more time to cook and overconsume, while those who were financially disadvantaged suffered from malnutrition and weight loss because of inflated food prices and food insecurity(17,18).
Recent research has linked obesity to an increased risk of contracting severe infections of COVID-19, thereby increasing the risk for extended hospitalisation and increased mortality(19). Importantly, therapeutic interventions and prophylactic treatments are more difficult and less effective in this group(20–25), with resultant poorer outcomes. Thus, weight gain secondary to pandemic confinement has an increased significance.
As the pandemic unfolds, researchers all over the globe try to better understand the prevalence, factors involved and impact of weight change in order to guide prevention strategies that will address this major public health crisis. These efforts have led to the identification of multiple determinants including biological, psychological and sociological processes that influence body weight during the pandemic. In this report, the interplay between these factors has been extrapolated from a systematic review of the current literature. Through an analysis of these observations, future public health interventions can be determined.
Materials and methods
Methods and analysis
This review has been informed by the Cochrane Handbook for Systematic Reviews of Interventions(26) and is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses(27). The review protocol is registered in the PROSPERO International Register of Systematic Reviews (Registration number CRD42020193440). This systematic review did not need approval from the ethics committee or required informed consent from the study populations as the data were retrieved from open-source databases and internet searches.
Search strategy
A medical librarian (L.Ö.) performed a comprehensive literature search in the electronic databases PubMed Embase, Scopus, PsycInfo, Cochrane, CINAHL and Web of Science in June and July of 2020. Search terms related to ‘pandemics’ AND ‘body weight’ AND ‘confinement’ were systematically developed with the help of PubMed and PubMed’s MeSH and reviewed and discussed with a subject specialist (M.A.B.K.). The search string developed in PubMed was later adapted and applied to all databases. A combination of the search fields of ‘Title’, ‘Abstract’ and MeSH/Thesaurus (when available) was used to ensure the best possible search precision and results. No filters or limitations were applied to ensure the inclusion of pre-indexed materials. All databases were searched from their inception until July 2020. Selected sources of grey literature and the preprint archive medRxiv were additionally included in the literature search. A search update in PubMed and Scopus was conducted on 1 February 2021. No additional relevant studies were located after hand screening the results from the updated search.
A search log with database specifications, detailed search strings, results and notes for all sources included in the search is available in online Supplementary Appendix 1.
Inclusion and exclusion criteria
All study designs relevant to human pandemic confinements and their effects on body weight were included (Table 1). All age groups were included, and there were no language restrictions.
Table 1.
Inclusion and exclusion criteria
| Inclusion | Exclusion | |
|---|---|---|
| Population | Human studies on pandemic confinement | Animal studies Studies investigating the effect of obesity or overweight on various outcomes during the pandemics Diseases such as HIV, measles and mumps |
| Effect | Studies describing the impact of quarantine on body weight Studies showing the impact of quarantine on the overweight/obese population |
Studies showing obesity or overweight as a risk factor for the pandemic |
| Outcome | Effect on body weight. Weight change (%), BMI change (kg/m2) Demographic, behavioural, social, physical, psychological, lifestyle and environmental behaviours during confinement that have effect body weight Studies showing measures taken to manage weight changes during confinement |
|
| Study | Designs: all study designs. Language: all languages. Year: publication year inception – 1 February 2021 |
This review was extended to articles published from the time of inception until 1 July 2020 and from an update in PubMed and Scopus on 1 February 2021. The primary outcome was to determine the effects of pandemic confinements on body weight. The secondary research outcome was to identify factors affecting body weight during pandemic confinements.
We excluded animal studies and studies investigating the effect of obesity or overweight on various outcomes during the pandemic. We also excluded studies that only narrated the effects of obesity or overweight as a risk factor worsening pandemic-related disease. Studies on diseases, such as HIV, measles and mumps, were also excluded.
Screening and selection
All references identified in the databases and grey searches (n 5070) were uploaded to the systematic review tool Covidence (Veritas Health Innovation, 2020) for automatic deduplication and blinded screening (Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram (Fig. 1)). Two reviewers (H.M. and M.A.B.K.) independently screened the references at both the title/abstract (n 2361) and full-text level (n 78). A third reviewer (P.M.) resolved any conflicts. The grey references and preprints were screened and deduplicated manually by M.A.B.K. and L.Ö. Finally, the reference lists of the included papers were hand screened. Those full-text articles that did not meet the inclusion criterion were excluded (n 27) (Fig. 1). One study investigating weight gain exclusively in pregnant women was excluded(28) as it was impossible to distinguish physiological from pandemic-related weight gain in this group.
Fig. 1.
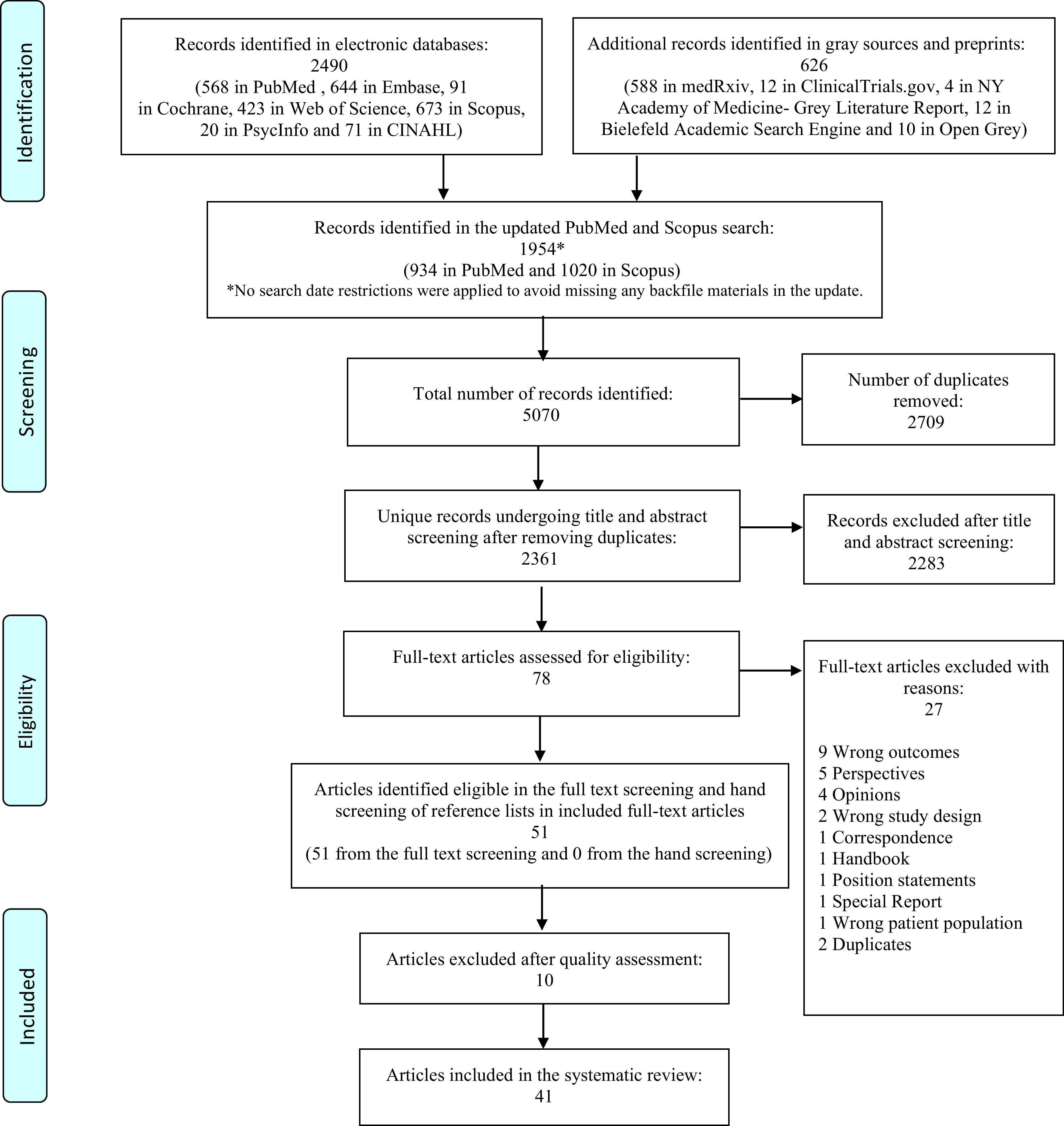
PRISMA flow chart showing the screening process.
Data extraction
The study characteristics including the authors, year of publication, country of origin, study design, research instruments used, validity of survey questionnaire, proportion of female participants, age range and mean age of participants, mean BMI of participants and mean weight of participants were extracted by one reviewer (M.A.B.K.). The other reviewers (P.M., R.G. and A.M.B.A.S.) extracted and reviewed the data independently. Determinants that had an impact on body weight were extracted and reviewed (primarily by M.A.B.K. and secondarily by P.M., R.G. and A.M.B.A.S.).
Quality assessment (n 51)
Two reviewers (M.A.B.K. and P.M.) independently performed a quality assessment of the fifty-one studies identified as eligible in the screening (online Supplementary Appendix 2). We applied a validated Newcastle–Ottawa Quality Assessment Scale to assess the quality of the studies that were included in the review(29–31). Quality scores obtained via the Newcastle–Ottawa scale for cross-sectional, cohort studies and case–control studies were used to assess selection, comparison and outcomes. Score disagreements were resolved through a discussion between M.A.B.K. and P.M., and a final consensual rating was assigned to each study. Studies six or more stars were considered high quality and were included in the review. Studies with fewer than six stars were excluded (online Supplementary Appendix 2).
Results (n 41)
Categorisation of determinants
Ten studies met the inclusion criteria covering pandemic confinements and their effects. These were then further subdivided into the following five main categories:
Demographic determinants
The impact of pandemic confinements on body weight
Dietary changes and other lifestyle behaviour changes during the confinement
Behaviour changes observed in obese participants
Determinants of obesity during pandemic confinements.
Our search yielded 5070 records of which 2361 unique studies remained after deduplication. After applying the inclusion and exclusion criteria in the title and abstract screening, seventy-eight articles were eligible for full-text screening (Fig. 1). We excluded ten studies based on a quality assessment of the results, and twenty-seven studies were excluded based on reasons presented in the Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram (Fig. 1). The range of observations covered dietary choices(13,20–25,32–35,35–46), lifestyle changes in children(23,35,47–50), physical activity levels(32–34,36,37–40,42,45,46,48,51,52–59), psychosocial factors(21,22,25,37,43,44,50,51,54,55,57,60,61), socio-economic factors(22,36,47,51,53,60) and sleeping patterns(25,45,50,62).
Demographic determinants (study and sample characteristics) (n 41)
Table 2 describes the characteristics of each of the forty-one included studies. All of the studies were published in 2020 and 2021. Two studies were from preprints and were included after assessing their qualities individually(21,51).
Table 2.
Characteristics of included studies
(Mean values and standard deviations)
| S. no | First author, year, country | Number of participants | Study design | Instrument used | Local setting/target population | Survey questions type | Proportion of female participants (%) | Age of participants range (years) | Mean age of participants (sd) | Mean BMI/Centile of participants |
Mean weight (kg) of participants | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Adıbelli, et al., 2020, Turkey(48). | 597 | Cross-sectional study | Online survey | Children aged 7–13 years and their parents | Validated | 55·8 | 7–13 (child) 26–57 (parent) |
9·87± 1·99 (children) 37·63 ± 5·83 (parents) |
NR | NR | |||
| 2 | Ahmed, et al., 2020, Iraq(60). | 765 | Prospective cross-sectional case series study | Face-to-face interview | Patients visiting bariatric clinic | Validated | 39·4 | < 20–> 70 | NR | NR | 73 | |||
| 3 | Athanasiadis, et al., 2020, USA(40). | 208 | Cross-sectional | Online survey | Postoperative bariatric patients | Validated | 86 | NR | 48·9 | 11·2 | NR | 92·1 | 23·6 | |
| 4 | Błaszczyk-Bebenek, et al., 2020, Poland(41). | 312 | Observational retrospective | Self-administered web-based questionnaire | Healthy adults | Validated | 64·1 | NR | 41·12 | 13·05 | 24·98 | 4·33 | 73·47 | 16·65 |
| 5 | Chagué et al., 2020, France(46). | 124 | Cross-sectional survey |
Phone interviews | Congestive heart failure patients | New | 39·5 | NR | 71·0 | 14·0 | 28·2 | 5·4 | ||
| 6 | Chopra, et al., 2020, India(54). | 995 | Cross-sectional study | Online survey | Adults ≥ 18 years | Validated | 41·5 | ≤30 > 30 | 33·33 | 14·5 | 24·8 ± 4·7 kg/m2 | NR | ||
| 7 | Cransac-Miet, et al., 2021, France(55). | 195 | Cross-sectional population-based study | Phone interview | Patients with chronic coronary syndromes | New | 39 | NR | 65·5 | 11·1 | NR | NR | ||
| 8 | Deschasaux-Tanguy et al., 2020, France(51). | 37 252 | Cross-sectional survey | Self-administered web-based questionnaire | NutriNet-Santé cohort | Validated | 52·3 | 18–80+ | 52·1 | 16·6 | NR | NR | ||
| 9 | Di Santo, et al., 2020, Italy(34). | 126 | Cross-sectional observational study | Telephone interview | Mild cognitive impairment patients | Validated | 81 | 60–87 | 74·29 | 6·51 | NR | NR | ||
| 10 | Di Renzo et al., 2020, Italy(20). | 3533 | Cross-sectional survey | Self-administered web-based questionnaire | General public | Validated | 75·1 | 12–86 | ± 13·53 | 27·66 | 4·10 | 66·87 | 13·16 | |
| 11 | Đogaš, et al., 2021, Croatia(63). | 3027 | Cross-sectional study | Online questionnaire | General public | Validated | 70·1 | NR | 40 | 30–50 | 74·03 | 16·03 | 24·64 | 4·22 |
| 12 | Dondi, et al., 2021, Italy(35). | 5811 | Cross-sectional study | Online survey | Italian resident parents of children ≤ 18 years | Validated | 91·7 | ≤ 30–> 50 | NR | NR | NR | |||
| 13 | Dragun, et al., 2020, Split, Croatia(50). | 531 | Cross-sectional study | Online survey | Students | Validated | 63·8 | 17–24 (median) | 18·0 | 6·0 | 21·4 | 3·3 | NR | |
| 14 | Drywień, et al., 2020, Poland(36). | 1769 | Cross-sectional study | Online survey | Polish women | Validated | 100 | ≥ 18 | NR | NR | NR | |||
| 15 | Dihogo Gama de Matos, et al., 2020, Brazil(58). | 426 | Cross-sectional study | Self-administered web-based questionnaire | General public | Validated | 49·1 | 7–80 | Multiple range from children to elderly | Multiple stratified per age | Multiple weight stratified per age | |||
| 16 | Gentile, et al., 2020, Vasto- Italy and Paraguay(61). | 110 | Observational study | Phone-based clinical follow-up and survey | Psychiatric outpatients |
Validated | 54·5 | NR | 38·6 | 14·1 | NR | NR | ||
| 17 | Giustino et al., 2020, Italy(52). | 802 | Cross-sectional study | Self-administered web-based questionnaire | Physically active participants | Validated | 51 | NR | 32·27 | 12·81 | 23·44 | 3·33 | 67·13 | 13·41 |
| 18 | He, et al., 2020, China(39). | 339 | Cross-sectional study | Online survey | Adults ≥ 18 years | New | 52·3 | NR | Males:36·4 (11·9) Females: 37·6 (12·4) |
NR | Female: 51·1 ± 4·1 Male: 65·6 ± 5·8 |
|||
| 19 | Ismail, et al., 2020, MENA Region(3). | 2970 | Cross-sectional | Online questionnaire | Adults ≥ 18 years | Validated | 71·6 | 18–> 55 | NR | NR | NR | |||
| 20 | Ismail, et al., 2020, UAE(33). | 1012 | Cross-sectional study | Online survey | Adults ≥ 18 years | Validated | 75·9 | 18– ≥ 36 | NR | NR | NR | |||
| 21 | Jia, et al., 2020, China(56). | 10 082 | Retrospective study | online questionnaire and | Chinese youth | Validated | 72 | 16–28 | 19·8 | 2·3 | 21·8 kg/m2 | NR | ||
| 22 | Jimenez, et al., 2021, Spain(43). | 603 | Cross-sectional study | Online survey | Patients attending obesity clinic | New | 27·5 | 18–≥ 55 | NR | 34·2 | 7·0 | NR | ||
| 23 | Kang, et al., 2021, Korea(49). | 226 | Retrospective cohort study | Retrospective review of medical records | Children followed-up at the growth clinic | Not applicable (anthropometric and laboratory parameters) |
57·5 | 4–18 | 10·5 (8·7–11·4) IQR | 0·2 (1·3) anthropometric z scores | 0·1 (1·2) anthropometric z scores | |||
| 24 | Karatas, et al., 2020, Istanbul(65). | 140 | Prospective observational case–control study | Physical and biochemical parameters | Known confirmed type 2 diabetes patients matched with healthy patients in outpatient clinic | None | Non-diabetic: 56·4 Diabetic: 68·2 |
NR | Non-diabetic: 52·61 ± 4·88 Diabetic: 54·81 ± 10·53 |
Total mean 31·63 ± 3·57 kg/m2
Non-diabetic: 31·63 ± 3·57 Diabetic 33·44 ± 6·48 |
Non-diabetic: 85·56 ± 10·53 Diabetic: 87·83 ± 18·27 |
|||
| 25 | Keel PhD, et al., 2020, USA(37). | 90 | Prospective study | Online surveys | Undergraduate psychology students | Validated | 88 | NR | 19·45 (1·26) years | 22·93 | 63·87 | |||
| 26 | Kriaucioniene, et al., 2020, Lithuania(38). | 2447 | Cross-sectional study | Self-administered web-based questionnaires | General public | Validated | 87·8 | > 18–≥ 51 | NR | NR | NR | |||
| 27 | Malkawi, et al., 2020, Jordan(57). | 2103 | Cross-Sectional Study | Online survey | Mothers living in Jordan who have at least one child between the ages of 4–18 years | Validated | NR | Mother’s age range: 20–60 years | 36·2 years | NR | NR | |||
| 28 | Marchitelli, et al., 2020, Italy(44). | 110 | Cross-sectional | Online survey | Day care patients in hospitals for obesity management | Validated | 71 | NR | No psychiatric illness: 18–75 years (M = 47·24, sd = 14·3) Psychiatric illness: 18–74 years (M = 46·38, sd = 14·5) |
No psychiatric illness: 40·19 kg/m2 (sd = 6·8, range: 27–60) Psychiatric illness: 39·88 kg/m2 (sd = 6·8, range: 28–55) |
NR | |||
| 29 | Martínez-de-Quel et al., 2021, Spain(45). | 161 | Longitudinal observational study | Online survey | Spanish adults | Validated | 37 | 19–65 | 35·0 | 11·2 | 23·7 | 4 | 67·3 | 14·8 |
| 30 | Mason, et al., 2021, USA(66). | 1820 | Longitudinal prospective cohort study | Online survey | High schools | Validated | 61 | NR | 19·28 | NR | 70·3 kg | |||
| 31 | Mitchell et al., 2020, USA(21). | 3 81 564 | Observational, retrospective, cohort study | Noom app – mobile behaviour change weight loss programme |
App-based food data from a digital behaviour change weight loss programme |
App-based validated | 83·4 | ≥ 18 | 47·76 | 13·59 | NR | 85·57 | 20·4 | |
| 32 | Önmez, et al., 2020, Turkey(64). | 101 | Retrospective observational study | Questionnaire | Diabetic patients attending polyclinics | Validated | 53·5 | 18–80 | 55 | 13 | 30·3 | 5·5 | 84·7 ± 16·4 kg | |
| 33 | Özden, et al., 2021, Turkey(42). | 1011 | Cross-sectional study | Online survey | Nursing students | Validated | 60 | NR | 19·97 ± 3·11 years | NR | NR | |||
| 34 | Pellegrini et al., 2020, Italy(22). | 150 | Observational retrospective study | Telephone interviews cross-sectional survey | Obese patients in weight loss programme | Validated | 76·3 | 18–75 | 47·9 | 16·0 | 36·6 | 4·5 | 92 | 17 |
| 35 | Pietrobelli et al., 2020, Italy(23). | 41 | Longitudinal observational study/questionnaire | In-person interview/telephone interviews (parents) | School children | New | 46·35. | 6–18 | 13 | 3·1 | 30·2 ± 4·1 a and BMI % Centile 98·2 ± 1·4 |
77·4 | 21·9 | |
| 36 | Rogers et al., 2020, UK(53). | 5820 | Cross-sectional survey | Self-administered web-based questionnaire | General public | Validated | 88 | 20–70+ | NR | NR | NR | |||
| 37 | Romero-Blanco et al., 2020, Spain(62). | 207 | Longitudinal observational study | Self-administered questionnaire | Nursing students | Validated | 81·6 | 17–53 | 20·6 | 4·62 | NR | NR | ||
| 38 | Ruissen, et al., 2021, Netherlands(59). | 435 | Observational cohort study | Online questionnaire | Type 1 and Type 2 diabetic patients | Validated | 42 | ≥ 18 | Type 1 DM: 50·1 (± 14·9) Type 2 DM: 62·5 (± 11·6) |
Type 1 DM: 25·9 (± 4·3) Type 2 DM: 30·2 (± 6·1) |
NR | |||
| 39 | Shah, et al., 2020, India(47). | 77 | Observational study | Follow-up in outpatient clinic | Children with type 1 diabetes | Validated | 58·4 | 5–20 | 14 ± 4 years | NR | NR | |||
| 40 | Sidor et al., 2020, Poland(24). | 1097 | Cross-sectional survey | Self-administered web-based questionnaire | General public | New | 95·1 | 18–71 | 27·7 | 9·0 | 21·5 23·5 | 4·8 | 66·0 | 14·5 |
| 41 | Zachary et al., 2020, USA(25). | 173 | Cross-sectional survey | Self-administered web-based questionnaire | General public | Validated | 55·5 | ≥ 18 | 28·1 | 12·5 | 27·0 | 7·6 | NR | |
BMI in children; NR, not reported.
The included studies had the following countries of origin: Brazil(58), China(39,56), Croatia(50,63), France(46,51,55), Jordan(57), India(47,54), Iraq(60), Italy(20,22,23,34,35,44,44,52), Korea(49), Lithuania(38), Netherlands(59), Poland(24,41,50), Spain(43,45,62), Turkey(42,48,64,65), United Arab Emirates(32), UK(53) and the USA(25,37,40,66). Furthermore, multi-regional studies conducted intercontinentally(21), among eighteen countries in the Middle East and North Africa region(33), and Paraguay and Italian-based multinational researches(61) are included in our analysis.
Altogether, the studies enrolled 469 362 participants. The participants ranged in age from 6 to 86 years, and the mean ages for the individuals studied ranged from 9·9 to 74·3 years. The proportion of female participants ranged from 37 % to 100 %. The number of participants in the included studies ranged from 41 to 381 564. All studies included both male and female participants except one study(36). The duration of confinement for the selected studies for this systematic review ranged between 1 and 24 weeks.
Impact of confinement on body weight
In our study, 7·2–72·4 % of all participants including both adults and children experienced an increase in body weight during the confinement periods(20,22,24,25,32,34–36,38–40,42–51,54,56–61,63–67)(Fig. 2). The mean weight gain ranged from 0·6 (sd 1·3) to 3·0 (sd 2·4) kg. There was a higher weight gain among participants who self-reported stress(25,44,54,55,57,60,61), anxiety and depression(22,51,57,60,61). Weight loss was observed in 11·1–32·0 % of participants(20,24,32,34,36,39,50,51,54,59,64,67). The mean experienced weight loss ranged from 2·0 (sd 1·4) to 2·9 (sd 1·5) kg.
Fig. 2.
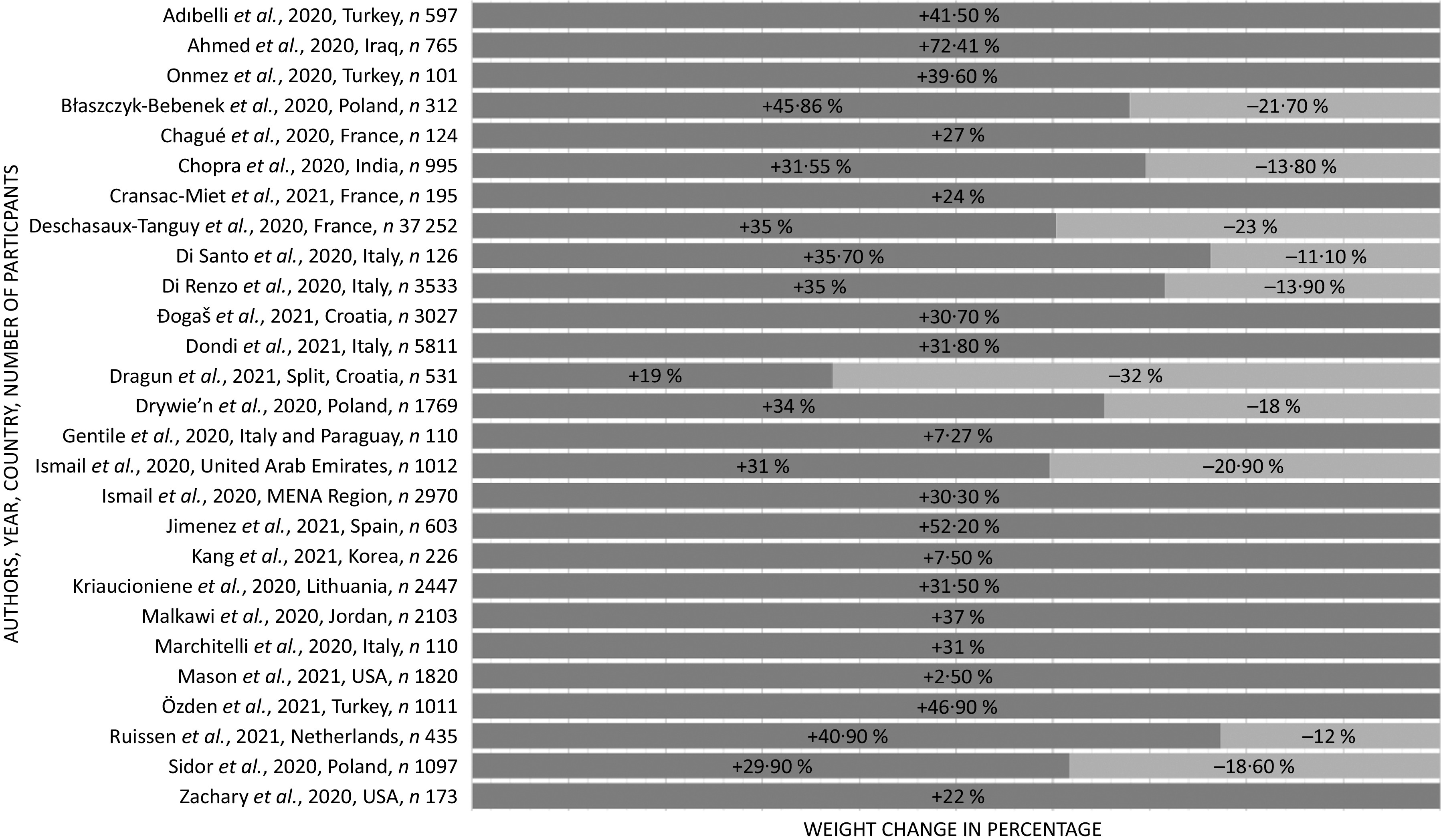
Body weight changes during pandemic confinements. Selected studies showing percentage of body weight changes. For the full list of weight changes, please refer to Table 3. +, increase in weight; −, decrease in weight.
Dietary and other lifestyle behaviour changes during confinement
Table 3 describes dietary and behavioural changes that were caused by pandemic-related confinements. Most studies reported an increase in food intake associated with increased snacking(20,22,24,25,32,33,35,36,38,40,42,43,50,51,54,67) and all these studies documenting perceived weight gain(20,22,24,25,32,33,35,36,38,40,42,43,50,51,54,67).
Table 3.
Behavioural and dietary changes related to pandemic confinements
| S. no | First author, year, country | Duration of confinement during which study was conducted (weeks) | Outcome area of focus | Number of participants | Weight gain of the participant (%) (weight gain in kg), weight loss of the participant (%) (weight loss in kg) |
Energy intake/food intake | Snacking | Fresh product (fruits and vegetable) | Physical activity | Alcohol | Dietary patterns and other behaviour changes identified during the confinement |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Adıbelli, et al., 2020, Turkey(48). | 4 weeks | Health related quality of life | 597 | ↑41·5 % | NR | NR | NR | NR | NR | Quality of life score mean 73·91 ± 8·44 Increase in sleep time of 34·2 % Increase Internet usage of 69·3 % |
| 2 | Ahmed, et al., 2020, Iraq(60). | 1–9 weeks | Body weight | 765 | ↑72·41 % | NR | NR | NR | NR | NR | One-third of them became emotionally unstable during the outbreak Even after the isolation process calmed down, the stress was present in more patients compared with the period of the outbreak |
| 3 | Athanasiadis, et al., 2020, USA(40). | 5 | Factors attributed to weight gain | 208 | 2 + 4·2 kg in patients > 18 months post-bariatric surgery | Increased | ↑62·6 % | ↓45·5 % | ↓55·2 % | ↑40·1 % | 19·5 % reported increase in binge eating 48·2 % reported loss of control when eating Weight gain of > 2 kg in patients > 18-month post-bariatric surgery |
| 4 | Błaszczyk-Bebenek, et al., 2020, Poland(41). | 5- 8 | Nutrition behaviour changes during lockdown | 312 | ↑45·86 % (0·56 ± 2·43 kg) ↓21·72 % |
↑11·2 % in number of meals | ↑from 72·8 % to 77·9 (P < 0·0001) | ↑from 63·8 % to 64·7 % (P = 0·7755) | NR | Increased | Increase of consumption of eggs, potatoes, sweets and canned meat |
| 5 | Chagué et al., 2020, France(46). | 6–7 | Impact of lockdown on health indicators and behaviours among congestive heart failure patients | 124 | ↑27·4 % | NR | NR | NR | ↓41·9 % | ↑4 % ↓15 % | Screen time increased by 46 %. Tobacco consumption increased in 44·4 % of current smokers. Adherence to strict salt and fluid restriction decreased by 14·5 %. Increase in heart failure symptoms in 21·8 % |
| 6 | Chopra, et al., 2020, India(54). | 20–22 weeks | Impact of COVID-19 on lifestyle-related behaviours: eating, physical activity and sleep behaviour | 995 | ↑31·55 % ↓13·87 % |
Increased | Increased | ↑ 34 v 38 % | ↓9·5 % | Decreased | In participants < 30 years old, increase in healthy food and restriction of unhealthy meals Increase stress amongst participants (25 % v 38·3 %) significantly increased Increase in daily sleeping hours, screen time, sitting time at work, stress levels. Decreased smoking |
| 7 | Cransac-Miet, et al.,2021, France(55). | 4 | Lifestyle changes | 195 | ↑24 % | NR | NR | NR | ↓ 25 % | 5 % increase in alcohol consumption | Smoking increased by 26 % Screen time increased in 65 % of patients |
| 8 | Deschasaux-Tanguy et al., 2020, France(51). | 2–6 | Changes in diet and physical activity during lockdown | 37 252 | ↑ 35 % (1·8 ± 1·3 kg), ↓23 % (2·0 ± 1·4 kg) | ↑10 % ↓10 % | ↑21·1 % | ↓10·1 % | ↑52·8 %, ↓ 18 % |
↑15 %, ↓ 12 % | Positive behavioural trends were observed in those with higher educational attainment with high income but negative trends were reported when income was lower Positive behavioural trends were observed in the overweight/obese population with higher educational attainment who expressed anxiety: reduced snacking: reduced alcohol consumption: increased more home cooking |
| 9 | Di Santo, et al., 2020, Italy(34). | 8–10 | Lifestyle, mental health Weight change Behavioural changes |
126 | ↑35·7 %, ↓11·1 % | ↑19·2 % | NR | NR | 1/3 of the subjects decreased their physical activity | Decreased in drinkers 12·4 % Increase in alcoholic drink intake (44·4 %) Two subjects started drinking wine |
1/6 of participants decreased mental-stimulating activities 70 % reported an increase in idle time 19·8 % were depressed, 9·5 % anxious and 9·5 % apathetic 31·9 % consumed more sweets 12·8 % ate preserved or frozen foods |
| 10 | Di Renzo et al., 2020, Italy(20). | 2–4 | Lifestyle changes, eating habits, and adherence to the Mediterranean diet during the COVID lockdown | 3533 | ↑ 35 %, ↓ 13·9 % | ↑ 34·4 % | ↑ 25·6 % | ↑ 37·4 %, ↓ 35·8 % | ↑38·3 % | NR | Younger participants adhered better to the Mediterranean diet Overweight participants had poor adherence to the Mediterranean diet 9·1 % of participants slept more than 9 h/d |
| 11 | Đogaš, et al., 2021, Croatia(63). | 2 | Lifestyle, mood | 3027 | ↑ 30·7 % | NR | NR | NR | Women decreased their exercise duration and frequency from 57·9 ± 34·5 to 51·1 ± 37·7 | Increased | Women smoked more cigarettes (P < 0·001) Increased frequency of feeling afraid (P < 0·001), discouraged (P < 0·001) and feeling sad (P < 0·001) in both sexes |
| 12 | Dondi, et al., 2021, Italy(35). | 24 | Perception of food insecurity in children | 5811 | ↑31·8 % | ↑27·3 % | ↑60·3 % | ↑ 14·0 % | NR | NR | 27·3 % Children were eating more food there was an increase. 60·3% consumption. 14 % fruit juices 10·4 % soft drinks. 2·5 % reported inadequate food after the pandemic |
| 13 | Dragun, et al., 2020, Split, Croatia(50). | 3–11 | Lifestyle changes and psychological state | 531 | ↑ 19 % ↓ 32 % |
No difference in dietary pattern | Increased 20–38 % | Increased (65·3 % v. 58·6 %) | Unchanged | NR | Improved sleep quality 31·5 %. Sleep hours increased Increased intake of legumes (60·6 % v. 53·3 %), fish (32·8 % v. 24·4 %) and sweets (30·5 % v. 22·4 %) Decreased intake of cereals (24·1 % v. 35·6 %), nuts (15·1 % v. 18·9 %), and dairy products Increase computer screen time due to online learning |
| 14 | Drywień, et al., 2020, Poland(36). | 3–7 | Changes in body weight due to COVID-19 lockdown | 1769 | ↑34 % ↓18 % |
↑65 % ↓40 % |
↑Salty snacks (30·4 % v. 11·3 %) | ↑Consumption of vegetables (32·3 % v. 16·0 %), fruit (23·8 % v. 14·3 %) in those with weight loss | ↓ In weight gainers (60·7 % v. 31·6 %) | ↑ In alcohol who gained weight (25·4 % v. 4·1 %) | Unhealthy dietary changes. Increase in screen time In females, those who lost weight ate more fruits, vegetable, pulses, seafood, drank > 500 ml water and did not consume alcohol Females who gained weight had increased consumption of sweetened spreads, commercial pastry, confectionery, salty snacks, fast food, sugar-sweetened beverages, processed meat, ice-cream and pudding and alcohol, decreased physical activity |
| 15 | Dihogo Gama de Matos, et al., 2020, Brazil(58). | 12 | Effects of COVID-19 social distancing on physical activity, stress levels, quality of life | 426 | Increased | NR | NR | NR | ↓84 % | NR | The study shows an overall decrease in all sections of quality of life as analysed by the SF-36. The elderly age group showed no significant changes. There has been increase in stress level across adolescents, adults and elderly age groups in both sexes (P < 0·05) although there is no difference of stress levels across children |
| 16 | Gentile, et al., 2020, Vasto-Italy and Paraguay(61). | 4–6 | Provide psychiatric assessments and measure the level of stress related to quarantine in a large sample of psychiatric outpatients | 110 | ↑7·27 % | NR | NR | NR | NR | ↑2·72 % | 56·3 % self- reported lifestyle changes during the confinement including: 32·7% eating pattern changes, 4·54 % Change in sleeping pattern, increased alcohol in 2·72 % Consumption, more reading and gaming in 16·3 %. Self-reported emotions from the patients ranked: Fear 24·5 % Optimism 20 % Pessimism 14·5 %, Hope 13·6 %, Hopelessness 10·9 %, Serenity, Anger 7·27 % |
| 17 | Giustino et al., 2020, Italy(52). | 1–2 | Changes in physical activity before and during the quarantine among the active Sicilian population | 802 | NR | ↓ 1168·5 MET – min/week | NR | NR | NR | NR | Greater impact of decreased physical activity among males and overweight participants |
| 18 | He, et al., 2020, China(39). | 4 | Body weight, physical activity and lifestyle changes | 339 | BMI < 24 gained weight Females: 2·2 kg Males: 1·7 kg |
Decreased | NR | NR | Decreased | Decreased | Weight correlated with the change level of alcohol consumption during the semi-lockdown for COVID-19 (Rs = –0·255; P = 0·002) |
| 19 | Ismail, et al., 2020, MENA Region(3). | 4–6 | Eating behaviours and lifestyle changes during COVID − 19 pandemic in Middle east and North Africa region (MENA) | 2970 | ↑30·3 % | Increased | 32·9 % had salty snacks | 48·8 % of surveyed participants did not consume fruits and 32·5 % did not consume vegetables daily | Increased level of inactivity from 34·9 % to 39·1 % | NR | Skipping meals decreased 74·0 % consumed less than eight cups of water per day. 44·1 % ate sweets or desserts |
| 20 | Ismail, et al., 2020, UAE(33). | 1–4 | Effect of quarantine on eating habits, physical activity, stress and sleep behaviours | 1012 | ↑31 % ↓20·9 % |
↑25·71 % ↓12·31 % |
37·1 % ate salty snacks | 48·8 % consumed fruits daily | ↑14·8 ↓41·9 |
NR | Increase in home cooked food, decrease in fast food consumption (P < 0·0001). Decrease frequency of meal skipping (64·5–46·2 %). Increase breakfast intake (66 % to 74·2 %). Increase water intake (24·1–27·8 %.) Main products consumed are sweets and desserts and salty snacks (chips, crackers, and nuts) during COVID-19 pandemic Inactivity levels rise (32·1–38·5%). 69·1 and 67·8 % of participants relied on social media applications to be updated about nutrition and health news |
| 21 | Jia, et al., 2020, China(56). | 11–14 | Activity performance and weight changes | 10 082 | BMI increased from 21·8 to 22·1 kg/m2
Overweight subject’s percentage increased from 21·4 % to 24·6 % Obesity participants percentage increased from 10·5 % to 12·6 % |
NR | NR | NR | Decreased (1·3–0·9 d/week, P < 0·001) |
NR | Increased sleeping hours (7·4–7·6 h/week, P < 0·001) on weekdays and (7·9–8·0 h/week, P < 0·001) on weekends Increase sedentary lifestyle (4·2–5·3 h/week, P < 0·001) on weekdays and (4·3–5·1 h/week, P < 0·001) on weekends Increased screen time (4·9–5·6 h/ week, P < 0·001) |
| 22 | Jimenez, et al., 2021, Spain(43). | 9 | Psychosocial, lifestyle and body weight effect due to COVID-19 lockdown | 603 | ↑52·2 | Increased | ↑19 % | ↑32·5 % | Decreased in > 50 % | Almost unchanged in 81·4 % (1·6 ± 1·2 v. 1·3 ± 0·7, P < 0·01) |
Patients with weight gain rated behavioural changes (4·1 ± 1·5 v. 2·5 ± 1·5, P < 0·01), physical activity (5·0 ± 1·4 v. 4·1 ± 1·6, P < 0·01), purchase of unhealthy and comfort foods (3·3 ± 1·6 v. 2·0 ± 1·2, P < 0·01), increase in consumption of sugary beverages (2·1 ± 1·5 v. 1·5 ± 1·0, P < 0·01) or alcohol (1·6 ± 1·2 v. 1·3 ± 0·7, P < 0·01), and snacking (3·6 ± 1·6 v. 2·1 ±1·3, P < 0·01). Bariatric surgery within 2 years acted as a protective factor against weight gain. Many experiences disordered sleep and mood |
| 23 | Kang, et al., 2021, Korea(49). | 24 | COVID-19 impact on childhood obesity and vitamin D levels | 226 | Overweight or obesity rate increased 23·9–31·4 % (7·5 % increase) | NR | NR | NR | Decreased due to school closure | NR | BMI z scores increased by 0·219 (P < 0·001) Increase level of TAG (105·8 mg/dL v. 88·6 mg/dl, P < 0·001) Increase level of LDL-cholesterol (100·2 mg/dl v. 94·0 mg/dl, P = 0·002). Decrease level of calcidiol level (18·9 mg/dl v. 23·8 mg/dl, P < 0·001). Patients who were normal weight had 9·9 (P < 0·001) times risk of becoming overweight or obesity during epidemic |
| 24 | Karatas, et al., 2020, Istanbul(65). | 24 | Body weight, metabolic control in type 2 diabetic patient and healthy population | 140 | Non-diabetic group (86·10 ± 10·48 v. 85·56 ± 10·53 kg) (P < 0·05) (0·54 ± 0·95) Diabetic group (89·75 ± 18·68 v. 87·83 ± 18·27 kg) (P < 0·05) 1·91 ± 5·48 kg |
NR | NR | NR | NR | NR | Non-significant change of BMI 33·44 ± 6·48 to 31·63 ± 3·57 kg/m2
HbA1c increased more in diabetic than in non-diabetic groups (P = 0·002) Glucose, LDL-cholesterol, and TAG increased in diabetic (39·69 ± 74·69, 7·60 ± 34·33, and 58·21 ± 133·54 mg/dl, P < 0·05) Waist circumference increased more in diabetic patients compared with non-diabetics (1·20 ± 2·38 v. 0·03 ± 0·46 cm, P < 0·05) TAG levels increased more in the diabetic group than in the non-diabetic group (P = 0·041) |
| 25 | Keel PhD, et al., 2020, USA(37). | 6–7 | Perceived v observed weight changes in undergrad students during COIVD-19 confinements |
90 | No statistically significant | Increased | NR | NR | Decreased | NR | Increase mean of weight description Increase screen time Increase time spent on TV Increased weight/shape concerns were significantly related to increased eating concerns Women had significantly higher weight/shape concerns than men Women at time 2 spent more time on social media compared with men |
| 26 | Kriaucioniene, et al., 2020, Lithuania(38). | 4 | Effect of COVID-19 on health behaviours and body weight | 2447 | ↑31·5 % | ↑49·4 | ↑45·1 % | ↓14·7 fruits ↓15·0 % vegetables |
69·9 % remained the same ↑14·2 % ↓15·9 % |
↓ 60·6 | 62·1 % cooked at home more frequently and (37·7 % increased the intake of homemade pastries while 26 % decreased intake of commercial pastries 20·6 % ate more fried food 41·3 % decreased fast food orders 19·4 % decreased carbonated and sugary drink intake Bought less manufactured pastries by 26 % |
| 27 | Malkawi, et al., 2020, Jordan(57). | 1–6 | Mental health and changes in lifestyle practices among Jordanian mothers during COVID-19 quarantine | 2103 | ↑37 % | NR | NR | 80·7 % consumed healthy diet | NR | NR | Increased teaching time of children Increased (63 %). Family violence Increased (27 %) hours spent in dedicated family time (+ 5 h). Mild levels of depression (mean = 11·5 ± sd = 9; range 0–42), anxiety (mean = 7·2 ± sd = 4; range 0–42), and stress (mean = 14·7 ± sd = 10; range 0–42). |
| 28 | Marchitelli, et al., 2020, Italy(44). | 9–11 | Weight gain in overweight/obese subjects Effect of psychological and psychosocial variables |
110 | Weight gain by 31 % of overweight/obesity Weight gain by 31 % of psychiatric patients |
60 % increased night eating | No significant changes | NR | NR | NR | Binge eating was significant factor for weight gain in psychiatric patients Increased night eating episodes in response to stress |
| 29 | Martínez-de-Quel et al., 2021, Spain(45). | 6–7 | Changes in physical activity, dietary habits and sleep quality pre- and post-lockdown | 161 | Pre 67·3 kg ± 14·8 v Post 67·7 kg ± 15·1 | NR | NR | NR |
↓8515·7 ± 10260·0 Met/ week v Post 5053·5 ± 5502·0 Met/ week p = <0·001 |
NR | Significant differences were found pre- and post-lockdown with physical activity sleep and perceived well-being, More people living together had higher weight gain |
| 30 | Mason, et al., 2021, USA(66). | 10–18 | Body weight change during lockdown and factors determining it | 1820 | Mean weight change 3·47 lbs (sd 14·57); mean % Weight change ↑ 2·5 % (8·6 %) |
↑31 % | NR | NR | NR | NR | 35 % consumed unhealthy food to cope with the pandemic Overeating as a mechanism of coping with pandemic was related to increase in weight and BMI on overweight |
| 31 | Mitchell et al., 2020, USA(21). | 1 | Alterations in food choices related to lockdown in users enrolled in a digital behavioural change weight loss programme | 381 564 | NR | NR | NR | ↓ 4·2 % | NR | ↓ 4·5 % | Use of the mobile app (Noom) decreased by 9 % |
| 32 | Önmez, et al., 2020, Turkey(64). | 15–24 | Glycaemic control in type 2 diabetes patients | 101 | ↑39·6 % ↓38·6 %, |
NR | NR | NR | Low: physical functioning on short form 36 – item survey (59·5 ± 26·9) |
NR | HbA1c increased from 7·67 ± 1·76 to 8·11 ± 2·48 compared with pre- and post-lockdown. The numbers of patients who exercised regularly and dieted were low. Mean pre-lockdown waist circumference was 105 ± 23 cm, compared with 107 ± 32 cm post-lockdown |
| 33 | Özden, et al., 2021, Turkey(42). | 8–10 | Nutrition exercise behaviours during lockdown | 1011 | ↑46·9 % | Increased | Increased | NR | ↓35·4 | NR | 26·8 % were bored. Psychological/addictive eating behaviour subscale scores were piled up between 20 and 40, and their unhealthy nutrition-exercise behaviour subscale mean scores were piled up between 30 and 50 (Fig. 1) |
| 34 | Pellegrini et al., 2020, Italy(22). | 4 | Weight and dietary changes before and during the COVID-19 lockdown in obese adults | 150 | ↑1·51 kg | ↑40 % | ↑33 % | ↓18 % | ↓ 60 % | NR | Increased weight gain with lower educational attainment and unhealthy food choices. Anxiety and depression increased weight gain by an average of 2·69 kg (95 % CI 1·66, 3·71; P < 0·001) |
| 35 | Pietrobelli et al., 2020, Italy(23). | 3 | Impact of COVID-19 lockdown on lifestyle factors in obese children | 41 | NR | ↑1·15 ± 1·56 meals per day | NR | NR | ↓2·30 ± 4·60 h/week | NR | Unhealthy food intake increased with significantly increased potato chips, red meat and sugary drink intakes during the lockdown (0·005 to < 0·001) Screen time increased by 4·85 ± 2·40 h/d Sleep time increased by 0·65 ± 1·29 h/d |
| 36 | Rogers et al., 2020, UK(53). | 2–4 | Altered physical activity among adults with serious health issues during COVID-19 lockdown |
5820 | NR | NR | NR | NR | ↑11·7 %, ↓ 25·4 % | NR | Being a female, living alone or not having access to a garden were also associated with less intensive physical activity |
| 37 | Romero-Blanco et al., 2020, Spain(62). | 4 | Sleep quality before and during the COVID-19 lockdown period in nursing students | 207 | NR | NR | NR | NR | NR | NR | Pittsburgh sleep quality index (PSQI) scored 0·91 points worse during the lockdown. Poor sleep incidence increased from 60·4 % to 67·1 % during the lockdown. Students with anxiety and depression had reduced sleep quality by 1·74 (0·85–2·63) points |
| 38 | Ruissen, et al., 2021, Netherlands(59). | 8–11 | Lockdown impact on people with type 1 and type 2 | 435 | ↑40·9 % ↓12 % |
NR | NR | NR | ↓45·7 % | NR | Increase in levels of stress 34·1 % Increase in levels of anxiety 27·3 % of all participants Stress correlated with poor glycaemic control (P < 0·0001) |
| 39 | Shah, et al., 2020, India(47). | 12–15 weeks | Glycaemic control, weight and BMI | 77 | Weight gain z score –0·4 ± 0·8 v. Post-lockdown weight z score –0·2 ± 0·8, P < 0·05) No significant increase in BMI |
↓ in low socio-economic state | Decreased | NR | NR | NR | Improved glycaemic control via HbA1C 79·4 ± 19·2 v. Post-lockdown Hba1C 74·5 ± 16·9 mmol/mol Improved glycaemic control in lower socio-economic state |
| 40 | Sidor et al., 2020, Poland(24). | 6 | Sleep quality before and during the COVID-19 lockdown period in nursing students | 1097 | ↑29·9 % (3·0 ± 1·6 kg), ↓18·6 % (2·9 ± 1·5 kg) | ↑43·5 % | ↑51·8 % | NR | NR | ↑14·6 % | Increased food consumption (55·3 %) and snacking (61·7 %) was reported by individuals with a higher BMI |
| 41 | Zachary et al., 2020, USA(25). | 4 | Diet choices and habits during COVID-19 lockdown | 173 | ↑22 % | ↑19 % | ↑63 % | NR | NR | NR | 73 % ate in response to boredom and 65 % in response to sight/smell of food Participants slept an average of 7·6 ± 1·3 h per night with less sleep related to weight gain |
↑, increased; ↓, decreased; NR, not reported; MET–minute/week, metabolic equivalent of task minute/week; IQR, interquartile range; lbs, pound.
Appetite was modified either negatively or positively and was associated with employment change, suspension or working from home(20,51,52,57) or due to suspension of school attendance(23,47–49).
The initiating factors were as follows: response to smell and sight of food(24,25), boredom, binge eating and food cravings(24,25,40,42,44,54,66), snacking post dinner(25,32,33,38) and visual stimulation through social media(32). A significant correlation was observed between snacking, the consumption of high density processed food and a higher BMI(20,22,24,38). Increased energy intake by 10–49·4 % was observed among study participants(20,22,24,25,32–38,40,42–44,51,54,66), particularly those with an increased consumption of high density processed foods(20,22,23,34–36,38,40,42,43,50,67), female sex(20,34,38,42,51,63) or with a higher BMI(20,22–24,36,38,43,44,48,63). There was an increase in the number of meals eaten per day(23,35,36,44,67) and participants ate more than usual(34,38,42,54). The proportion of respondents engaged in cooking increased from 40 % to 62 % in our study sample(24,32,38,51). Likewise, consumption of homemade recipes increased(22,23,32,33,38,51) and eating homemade desserts increased compared with pre-lockdown(20,22,23,32,34,38,67).
Less than one-third of the surveyed participants consumed fresh fruits and vegetables on a daily basis, while a similar number consumed sweets and desserts every day(20,21,24,32,36,38,39,51). In contrast, some studies have shown a decrease in unhealthy food consumption(22,33,38,54,67).
Where Mediterranean diet was followed, 18- to 30-year-olds were more compliant than other age groups(20). Inverse associations were found between adherence to Mediterranean diet and BMI(20,50). A total of 54 % of respondents used leftovers for at least a third of meals, and those who shopped at farmers’ markets or local or organic markets ate up leftovers more (OR = 1·468, P < 0·001)(20). Among app users, mobile behavioural change app interaction was reduced by 9 %(21). Eating in response to stress was associated with weight gain(25,34,58,66). There was increased alcohol consumption(24,46,55,61,67) during the lockdown, while a decrease in alcohol consumption was also noted compared with pre-COVID-19 in another study(20,46). There was an increase in cigarette smoking generally(46,50,55,63) while in contrast, 3·3 % of the smokers surveyed reported reduced smoking during quarantine(19).
Although the participants reported spending more time in bed before lockdown(23,25,54,56), the overall sleep quality was worse(45,54,62). In contrast, secondary school students felt refreshed on awakening and increased sleeping hours(50). Weight gain was reported by others to be related to decreased night-time sleep and reduced physical activity time(25,40,59)
Sedentary lifestyle and screen time increased during the lockdown(23,25,37,46,54–56). Those participants who were not currently working or those who started working from home felt that they gained more weight compared with participants who did not have a change in job routine(20,20,51,57).
Physical activity altered by varying amounts, reduced in some studies to between 18 and 84 %(22,23,33,34,39,46,51,53,54,58). People who were already overweight or obese engaged in less physical activity and had decreased energy expenditure during lockdown(36,38,43,51–53,55,58). Obese children spent less time participating in sports activities(23).
By contrast, studies reporting an increase in physical activity(20,51) found greater engagement in yoga/pilates, functional training, home training, and treadmill use and overall increased training frequency(20).
Behaviour changes observed in obese participants
Weight gain was more common in those already overweight or obese prior to lockdown and in individuals with pre-existing difficulty in weight management(20,22–24,36,38,43,45,48,63).
Increased snacking and food consumption were observed in participants with a higher BMI(23,24,32,33,67). Many of the participants agreed that they consumed less fruits and vegetables on a daily basis(21,24,33,38,40,51) but more high energy processed foods(22–24,40,43).
This intake was associated with an enhanced appetite and after-dinner hunger(20,36,38,44). Obese children reported an increase in the number of meals eaten along with an increased consumption of sweetened drinks, potato chips and red meat(23). A decrease in intensive physical activity was associated with obesity(53). An inverse relationship was found between changes occurring in sporting activities and the number of meals consumed per day(23,40,52,67). The participants self-reporting anxiety and depression displayed an estimated weight gain(22,44,54,55,57,61).
Determinants that can influence body weight during pandemics
Table 4 describes the determinants of body weight changes during the pandemic. Many determinants that can influence increased weight gain during confinement were identified via this current systematic review. This includes past behaviours, dietary behaviours, physical activity patterns, work environment, psychosocial and socio-economic factors, and pre-existing co-morbidities.
Table 4.
Determinants of body weight during pandemic confinements
| Determinants that can influence weight gain |
|---|
| Demographics |
| Female(21,34,39,43,52,64) |
| Baseline obese and overweight(21,23,24,25,37,38,42,44,49,64,67) |
| BMI < 24(36,49) |
| Age group > 45 years(24,38,53) |
| Age group < 25 years(24,40) |
| Having children under the age of 18 at home(51) |
| Changed work environment to working from home(20,40,57) |
| Work environment |
| Loss of job(20) |
| Interruption of work routine(20) |
| Changed work habits: furloughed or working from home(20) |
| Suspension of schools(48) |
| Dietary behaviours |
| Increased food consumption(23,33,34,37,38,39,44,67) |
| Decreased consumption of fresh food products (particularly fruits, vegetables and fish)(20,21,36,38,39) |
| Increased consumption of homemade recipes, sweets and pizza(22,23) |
| Increased home cooking(38) |
| Increased cereal consumption(20,22) |
| Consumption of unhealthy foods(21,23,24,34,37,39,41,43,44,51) |
| Poor attention to diet balance(22) |
| Snacking after dinner(20,25) |
| Binge eating(40,44) |
| Loss of control to eating(40) |
| Eating in response to stress as a coping mechanism(21,25) |
| Eating secondary to appearance and smell of food(25) |
| Emotional eating(21,42,44) |
| Increase in alcohol intake(34,36,38) |
| Psychological factors |
| Decreased sleep time(25,43) |
| Lower sleep quality(45,62) |
| Stress(22,26,44,55,56,58,59,61) |
| Boredom(22) |
| Living alone(22,34) |
| Anxiety/depression(23,58,41,61,64) |
| Depressive symptoms(34,40,44) |
| Mood disturbances(43) |
| Weight/shape concerns(37) |
| Socio-economic factors |
| Lack of garden(53) |
| Urban residence(51,60) |
| Lower education level(22,57) |
| Residence in a macroeconomic region > 50 % of EU-28 GDP(36) |
| Lower socio-economic level(47,57) |
| Physical inactivity |
| Physical activity before lockdown(52) |
| Decreased physical activity(23,24,26,33,34,37–41,44,43,46,47,49,53,55,56–60) |
| Limitations of outdoor and in-gym activities(20,22,34,42) |
| Increased screen/TV time(23,37,38,47,55–57) |
| Co-morbidities |
| Associated chronic illness(47,52,56,65,66) |
| Determinants that can be associated with weight loss |
| Underweight before confinement(24,36) |
| Younger age(36) |
| Remote work(36) |
| Urban residence(51,60) |
| Ate less(36) |
| Ate more fruits/vegetable(36) |
| Drank more water(36) |
| Ate more pulses/seafood/fish(36) |
| Did not consume alcohol(36) |
| Regular exercise before lockdown(63) |
Female sex(20,34,38,42,51,63), age under 25 years and over 45 years(24,38,51,53) are in particular at higher risk of gaining weight. Initial weight status, diet quality and physical exercise pattern before lockdown are important factors(20,22–24,36,44,48,51,63). In Chinese(39) and Korean(49) populations, BMI < 24 kg/m2 was associated with weight gain. However, some observed that those who were underweight before confinement lost more weight during confinement(24,36).
Poor diet quality before the lockdown was associated with weight gain(51). Decreased consumptions of legumes, fruits and vegetables(24,38) were related to an increased consumption of sweets(22–24). Moreover, more home cooking with consumption of unhealthy foods is associated with increased weight gain(19,21,22,33–35,37,39,41,42,47,64) as is increased alcohol intake(34,36,38,46,67).
Less intense physical behaviours were noted during lockdown periods compared with behaviours before lockdown causing increased weight gain(22,23,25,32–34,36–40,42,45,46,48,52,54–59). This was due to the limitations of outdoor activities and in-gym activities(20,42,52). In addition, there has been more sedentary behaviour with increased screen time(22,36,37,46) which has been associated with weight gain.
Changed working habits, whether furloughed or working from home during the lockdown or those who had their job suspended(20,51,57), having children aged < 18 years at home(51), urban residence and attaining a lower educational level(22) were associated with weight gain.
Patients with pre-existing psychiatric co-morbidities had weight gain during COVID-19 lockdown(34,37,43,44,51,61), and stress(21,25,44,54,55,55,57,60,61), anxiety and/or depression(22,57,60,61), eating in response to stress(21,25), boredom(22), living alone(22), emotional eating(21,42,44) or weight or body shape concerns(37) were associated with an increase in body weight during confinement. Decreased sleeping time(25) or poor quality sleep(45,50,62) was further associated with weight gain.
Socio-economic factors such as urban residence(51,60), lack of access to garden(53), lower socio-economic level(47) or lower education levels(22) and residence in a macroeconomic region(36) were associated with significant gain in the weight.
Patients with chronic illness such as diabetes, hypertension, lung disease, chronic CHD, congestive heart failure, depression or disability affecting one or more activities of daily living or lower levels of physically activity had an increase in weight(34,40,44,46,51,53,55,61,64).
Those who were previously underweight before the lockdown tended to lose more weight(24,24,36). Those whose diet included more fruits and vegetables, pulses and drank more water lost weight(36).
Discussion
This systematic review highlights contrasting effects of pandemic confinements on body weight, and we identified specific factors associated with change in body weight during the lockdown periods.
A BMI of > 25 kg/m2 was identified as an independent risk factor for increased food intake during lockdown(68). Other influences were inadequate sleep, decreased physical activity, emotional eating in response to stress, lack of control in dietary habits(20,24,53) and increased alcohol consumption and smoking(34,36,38,50,55,63,67). The impact of these influences is more significant in the obese population.
Eating habits as well as diet composition are linked to weight gain(69). Increased snacking after meals, particularly post dinner, was associated with weight gain(69). Jakubowicz et al. also concluded that increased energy content at dinner increased the subjects’ weight(70). Thus, decreasing food consumption during and post dinner should be recommended.
Social networks, neighbourhood social activities and physical activity can influence an individual’s opportunity to make better choices contributing to protection from obesity(71). The absence of these influences during extended lockdown periods may facilitate a more obesogenic environment, thus encouraging weight gain(72).
By contrast, not all effects of pandemic confinement resulted in weight gain. In an Italian study, 38 % of participants adhered to a Mediterranean diet. This may have been assisted by the Italian Ministry of Health publishing online materials regarding favourable lifestyle choices during the lockdown in April 2020 and providing practical guidelines on healthy behaviours(73,74).
Pandemic confinements undoubtedly increase stress(33,55,58–60), 73 and 83 % of respondents experienced an increase in anxiety and depression, respectively, with 70 % reporting weight management issues, stock-piling food and stress eating(22,40,75). Weight loss was reported in three studies by 13–19 % of participants(20,24,51). Two studies showed stress-related weight among working professionals and university students(76,77). The mechanism is twofold and results from decreased, unchanged or increased energy intake coupled with adaptive adrenergic stimulated thermogenesis involving brown adipose tissues(78). The weight loss observed in this systematic review may also be attributed to the negative effect of stress(20,24,25,51,79).
The link between weight changes and stress has been studied extensively(80,81). Behavioural and physiological explanations suggest that the sensation of eating is associated with a psychological escape from emotional distress(82) and that the consumption of high energy foods alleviates stress(80). During a pandemic, where cities and even entire nations were locked down, fear and anxiety related to COVID-19 induced an over eating behaviour. However, management of this associated condition is difficult(83). The adverse effects of lockdown on the psychological and social well-being of society emphasise the need for strong public health interventions to support particularly at-risk people.
The associations between health outcomes, exercise and physical activity are well-established. The results from studies that we included in this review were mixed; some participants engaged in increased physical activity, while others had lower levels of physical activity. Confinement did not induce many sedentary participants to increase their physical activity. Other unhealthy behaviours such as increased screen time were noted which are similar to previous studies(84). Stress may impair efforts to become physically active; conversely, those who already participate may do so to reduce stress(85), which may explain the variation in physical activity observed. Seigel et al. describe this as stress-related behavioural activation or inhibition(86).
Other unhealthy behaviours were noted during the confinement. There was a 14·6 % increase in the consumption of alcohol in participants who had issues with alcohol(24). In the acute post-disaster period of the September 11 attacks in Manhattan, New York City, the prevalence of alcohol consumption and marijuana use among New York City residents increased over a 5–8-week period(87). These results mirror our findings, suggesting shared responses to intense community stresses. Although these activities may not directly affect weight, alcohol consumption and obesity are common risk factors for chronic illnesses leading to increased morbidity and mortality(88). Furthermore, in a study conducted in the Netherlands, it was reported that overweight and obese individuals found it more difficult to make healthy food choices. More savoury snacks and non-alcoholic beverages were purchased and consumed at home (35·6 %) because of more leisure time (31·5 %) and boredom (21·9 %) during the lockdown(89).
Positive outcomes from confinement have also been reported(90). These behaviours may result from the increased availability of time to cook, health risk perceptions, lack of negative social distractions(91) and socio-cognitive ideation towards a healthier lifestyle(92). Long-term studies are necessary to determine whether these constructive and preventive behaviours can be sustained after confinement is over.
Food security, which involves food availability, accessibility and affordability, is another important factor in the relationship between pandemic confinement and body weight changes(93). Global non-pharmaceutical interventions, such as lockdowns and quarantines, implemented to limit the spread of the virus have seriously impacted food security systems(94), with the greatest burden affecting communities in which nutritional health is fragile(95). Communities with precarious budgeting practices were destabilised by food price inflation and product shortages. Additional influences on food security included movement restrictions of workers, changes in consumer demand, closure of food production facilities, restricted food trade policies and financial pressures in the food supply chain. As dependence on food banks grew with an exponential increase in demand, basic survival needs presided over healthy dietary choices(17). Prior to 2020, 690 million people were already food insecure and hungry(96). By the end of 2020, the COVID-19 pandemic had created an additional 270 million food-insecure people(97,98). Unfortunately, vulnerable populations are not restricted to under-resourced countries; developed nations are suffering as well. In the USA alone, food insecurity more than doubled as a result of the economic crisis brought on by the outbreak, impacting as many as 23 % of households(99).
Serious ethical and health-related issues hinder healthcare providers working with vulnerable populations. In general, differences in weight status and dietary intake reveal that a trend in obesity increases as the degree of food insecurity increases(100). The COVID-19 crisis has highlighted food insecurity as a significant factor in nutritional poverty(94). This awareness of food insecurity may provide nations with the impetus to robustly tackle food-related epidemics, such as obesity and diabetes.
COVID-19 has challenged us to consider the role and balance of healthcare, personal health and holistic well-being. Redefining these dynamics in preparation for future pandemics is imperative to minimise severe impacts to health and resources(101). It was previously observed that consumerism is affected by internal factors, such as personal character, and external factors, such as economic crises. The pandemic served as an external factor that altered consumer behaviour(102).
Relief efforts by governmental and non-governmental agencies achieved temporary solutions without significant public pressure(103), but the demand for aid from all sectors of society is mounting. National governments should take the lead in providing strategic directions that will ensure the continuity of food accessibility to all, particularly the most vulnerable. Focus must be on coordinated and integrated public health programmes through legislative action to end sub-standard dietary conditions endured by those most in need. By collaborating with key stakeholders, health professionals must provide aggressive nutritional counseling to improve dietary habits, and concerted efforts across the board are paramount.
Recent research has shown obesity to be an independent risk factor for severe complications and increased mortality from COVID-19(104,105). The evidence suggests a linear relationship with obesity increasing the risk of severe disease and death among COVID-19 patients(106). The co-existence of both pandemics, COVID-19 and obesity, along with the emergence of obesity evolving from lockdown has caused a ‘syndemic’ or a symbiotic pandemic(107). Researchers must address the significant knowledge gaps that have become apparent during this pandemic regarding preparedness and response to such a crisis. Moreover, COVID-19 has disproportionately affected certain populations, and future research should focus on such vulnerable populations to ensure better outcomes.
Strengths and limitations
To our knowledge, this is the first systematic review evaluating the effects of pandemic confinement on body weight. Our study highlights major determinants that can have an impact on body weight during confinement and those that can be targeted in future pandemics to effectively manage body weight during pandemics via public health initiatives. Moreover, confinements are not solely related to pandemics and can also occur during natural disasters or calamities and in prisons. Determinants identified could be modified via appropriate public health measures to reduce negative impacts.
The present study has limitations. First, there was limited evidence from past pandemics related to obesity and morbidity or mortality. This may reflect the recent evolution of worldwide obesity(108). Second, within the common research theme of body weight changes during pandemic confinements, our systematic review found marked heterogeneity in the determinants and measured outcomes. This variation could be explained by differences in the study population and types of outcome measurements(109). Nevertheless, in our systematic review, we followed a rigorous protocol with clear objectives and inclusion and exclusion criteria. This allowed for the identification and pooling of the determinants of body weight changes during pandemic confinements (Table 4). A thorough and complete identification of the different determinants related to pandemic confinements could guide decision makers. Furthermore, our study calls for further research into the level of impact of each determinant. Third, given the contemporary nature of the pandemic, the literature was primarily related to countries where COVID-19 had an early ‘first wave’ impact. Findings from other continents, particularly from Africa and South America, are yet to emerge. Fourth, online surveys using social media platforms were the predominant data collection method, which has recognised strengths and biases. Although the researchers used this form of data collection to reach a wider population, the likelihood of a bias towards a younger population should be noted. Fifth, although this analysis provides evidence for the effects of confinement on body weight, we are unable to comment on the potential for interventions such as lifestyle changes to attenuate the phenomenon. Sixth, because of the limited number of studies included, we were unable to correct for influences, such as pre-existing diets, and could not quantify the impact of possible factors in isolation. Although we know that weight gain is likely during confinement, further research using more sophisticated data collection techniques is necessary to determine the holistic impact of confinement to provide evidence-based practical solutions for future eventualities.
Conclusion
This systematic review highlights the significant effects that pandemic confinements can have in the short term on body mass. Poor sleep, snacking post dinner, lack of dietary restraint, pre-existing overweight status, emotional eating due to stress and decreased physical activity are risk factors for weight gain.
Preparing for the next ‘wave’ is challenging given the multitude of factors that must be tailored to the local situations and available resources. Planning for future episodes requires a strong, evidence-based national policy in conjunction with clear guidelines to ensure that the negative sequelae of lockdowns are minimised.
Acknowledgements
We thank Gamila Hassan at the National Medical Library at UAEU for her strategic support in locating and uploading full-text articles to Covidence.
The authors received no specific funding for this work.
M. A. B. K., P. M., R. G., L. Ö. and H. M. formulated the research question and designed the study. M. A. B. K., P. M., R. G., K. K. A. and A. M. B. A. S. extracted and reviewed the data independently. L. Ö. and M. A. B. K. performed the literature search. M. A. B. K., P. M., R. G., A. M. B. A. S. and J. N. performed the literature review and data analysis. M. A. B. K., P. M., K. K. A., H. M., R. G, A. M. B. A. S., J. N., L. Ö., J. E. M. S. and J. K. contributed to drafting the paper. M. A. B. K., R. G., P. M., A. M. B. A. S. and K. K. A. equally contributed to all of the work as co-first authors.
We declare that there are no conflicts of financial and commercial interest that could be perceived as prejudicing the impartiality of the present study.
This article was not plagiarised and had not previously been published in other journals.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114521000921.
click here to view supplementary material
References
- 1. CDC COVID-19 Response Team (2020) Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep 69, 343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madhav N, Oppenheim B, Gallivan M, et al. (2017) Pandemics: Risks, Impacts, Mitigation. Disease Control Priorities: Improving Health, Reducing Poverty, 3rd ed. Washington, DC: The International Bank for Reconstruction and Development/The World Bank. [PubMed] [Google Scholar]
- 3. Ismail L, Materwala H, Znati T, et al. (2020) Tailoring time series models for forecasting coronavirus spread: case studies of 187 countries. Comput Struct Biotechnol J 18, 2972–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khan MA & Moverley Smith JE (2020) “Covibesity,” a new pandemic. Obesity Med 19, 100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al Falasi RJ & Ab Khan M (2020) The impact of COVID-19 on Abu Dhabi and its primary care response. Aust journal general practice 49. [DOI] [PubMed] [Google Scholar]
- 6. Every-Palmer S, Jenkins M, Gendall P, et al. (2020) Psychological distress, anxiety, family violence, suicidality, and wellbeing in New Zealand during the COVID-19 lockdown: a cross-sectional study. PLoS One 15, e0241658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mozaffarian D, Hao T, Rimm EB, et al. (2011) Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 364, 2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan MA, Hashim MJ, Mustafa H, et al. (2020) Global epidemiology of ischemic heart disease: results from the global burden of disease study. Cureus 12, e9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gallo L, Moritz K & Akison L (2020) Nutrient intake, physical activity levels, and metabolic status in Australian university biomedical students. Curr Dev Nutr 4, 1404–1404. [Google Scholar]
- 10. Tsenoli M, Moverley Smith JE & Khan MA (2021) A community perspective of COVID-19 and obesity in children: causes and consequences. Obesity Med 22, 100327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diaz-Zavala RG, Castro-Cantú MF, Valencia ME, et al. (2017) Effect of the holiday season on weight gain: a narrative review. J Obes 2017, 2085136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rundle AG, Park Y, Herbstman JB, et al. (2020) COVID-19-Related school closings and risk of weight gain among children. Obesity 28, 1008–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mason F, Farley A, Pallan M, et al. (2018) Effectiveness of a brief behavioural intervention to prevent weight gain over the Christmas holiday period: randomised controlled trial. BMJ 363, k4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hafner JW, Hough SM, Getz MA, et al. (2010) All-terrain vehicle safety, use patterns in Central Illinois youth. J Rural Health 26, 67–72. [DOI] [PubMed] [Google Scholar]
- 15. Pearl RL (2020) Weight Stigma and the ‘Quarantine-15’. Obesity 28, 1180–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laborde D, Martin W, Swinnen J, et al. (2020) COVID-19 risks to global food security. Science 369, 500–502. [DOI] [PubMed] [Google Scholar]
- 17. Huizar MI, Arena R & Laddu DR (2020) The global food syndemic: the impact of food insecurity, Malnutrition and obesity on the healthspan amid the COVID-19 pandemic. Prog Cardiovasc Dis 64, 105–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The Lancet Global Health (2020) Food insecurity will be the sting in the tail of COVID-19. Lancet Glob Health 8, e737–e737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cuschieri S & Grech S (2020) Obesity population at risk of COVID-19 complications. Glob Health Epidemiol Genom 5, e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Renzo L, Gualtieri P, Pivari F, et al. (2020) Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Translational Med 18, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mitchell ES, Yang Q, Behr H, et al. (2020) Self-reported food choices before, during COVID-19 lockdown. MedRxiv. doi: 10.1101/2020.06.15.20131888 [DOI] [Google Scholar]
- 22. Pellegrini M, Ponzo V, Rosato R, et al. (2020) Changes in weight and nutritional habits in adults with obesity during the ‘lockdown’ period caused by the COVID-19 virus emergency. Nutrients 12, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pietrobelli A, Pecoraro L, Ferruzzi A, et al. (2020) Effects of COVID-19 lockdown on lifestyle behaviors in children with obesity living in Verona, Italy: a longitudinal study. Obesity 28, 1382–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sidor A & Rzymski P (2020) Dietary choices and habits during COVID-19 Lockdown: experience from Poland. Nutrients 12, 1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zachary Z, Brianna F, Brianna L, et al. (2020) Self-quarantine, weight gain related risk factors during the COVID-19 pandemic. Obes Res Clin Pract 14, 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JP, Thomas J, Chandler J, et al. (2019) Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151, 264–269. [DOI] [PubMed] [Google Scholar]
- 28. Zhang J, Zhang Y, Huo S, et al. (2020) Emotional eating in pregnant women during the COVID-19 pandemic and its association with dietary intake and gestational weight gain. Nutrients 12, 2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herzog R, Álvarez-Pasquin MJ, Díaz C, et al. (2013) Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 13, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Modesti PA, Reboldi G, Cappuccio FP, et al. (2016) Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One 11, e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peterson J, Welch V, Losos M, et al. (2011) The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute. [Google Scholar]
- 32. Cheikh Ismail L, Osaili TM, Mohamad MN, et al. (2020) Eating habits and lifestyle during COVID-19 lockdown in the United Arab Emirates: a cross-sectional study. Nutrients 12, 3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ismail LC, Osaili TM, Mohamad MN, et al. (2020) Assessment of eating habits, lifestyle during coronavirus pandemic in the MENA region: a cross-sectional study. Br J Nutr 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Di Santo SG, Franchini F, Filiputti B, et al. (2020) The effects of COVID-19, quarantine measures on the lifestyles, mental health of people over 60 at increased risk of dementia. Front Psychiatr 11, 578628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dondi A, Candela E, Morigi F, et al. (2021) Parents’ perception of food insecurity and of its effects on their children in Italy six months after the COVID-19 pandemic outbreak. Nutrients 13, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drywień ME, Hamulka J, Zielinska-Pukos MA, et al. (2020) The COVID-19 pandemic lockdowns and changes in body weight among polish women. A cross-sectional online survey Plifecovid-19 study. Sustainability 12, 7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Keel PK, Gomez MM, Harris L, et al. (2020) Gaining “The Quarantine 15:” Perceived versus observed weight changes in college students in the wake of COVID-19. Int J Eating Disorders 53, 1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kriaucioniene V, Bagdonaviciene L, Rodríguez-Pérez C, et al. (2020) Associations between changes in health behaviours and body weight during the COVID-19 quarantine in Lithuania: the Lithuanian COVIDiet Study. Nutrients 12, 3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. He M, Xian Y, Lv X, et al. (2020) Changes in body weight, physical activity, and lifestyle during the semi-lockdown period after the outbreak of COVID-19 in China: an online survey. Disaster Med Public Health Prep 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Athanasiadis DI, Hernandez E, Hilgendorf W, et al. (2020) How are bariatric patients coping during the coronavirus disease 2019 (COVID-19) pandemic? Analysis of factors known to cause weight regain among postoperative bariatric patients. Surg Obes Reltd Dis 17, 756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Błaszczyk-Bębenek E, Jagielski P, Bolesławska I, et al. (2020) Nutrition Behaviors in Polish Adults before and during COVID-19 Lockdown. Nutrients 12, 3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ozden G & Parlar Kiliç S (2021) The effect of social isolation during COVID-19 pandemic on nutrition and exercise behaviors of nursing students. Ecol Food Nutr 1–19. [DOI] [PubMed] [Google Scholar]
- 43. Jimenez A, de Hollanda A, Palou E, et al. (2021) Psychosocial, lifestyle, body weight impact of COVID-19-Related lockdown in a sample of participants with current or past history of obesity in Spain. Obes Surg 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marchitelli S, Mazza C, Lenzi A, et al. (2020) Weight gain in a sample of patients affected by overweight/obesity with and without a psychiatric diagnosis during the Covid-19 lockdown. Nutrients 12, 3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martínez-de-Quel Ó, Suárez-Iglesias D, López-Flores M, et al. (2021) Physical activity, dietary habits and sleep quality before and during COVID-19 lockdown: a longitudinal study. Appetite 158, 105019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chagué F, Boulin M, Eicher J-C, et al. (2020) Impact of lockdown on patients with congestive heart failure during the coronavirus disease 2019 pandemic. ESC Heart Fail 7, 4420–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shah N, Karguppikar M, Bhor S, et al. (2020) Impact of lockdown for COVID-19 pandemic in Indian children, youth with type 1 diabetes from different socio-economic classes. J Pediatr Endocrinol Metab 1. [DOI] [PubMed] [Google Scholar]
- 48. Adıbelli D & Sümen A (2020) The effect of the coronavirus (COVID-19) pandemic on health-related quality of life in children. Children Youth Serv Rev 119, 105595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kang HM, Jeong DC, Suh B-K, et al. (2021) The impact of the Coronavirus Disease-2019 pandemic on childhood obesity, vitamin D status. J Korean Med Sci 36, e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dragun R, Veček NN, Marendić M, et al. (2021) Have Lifestyle Habits and Psychological Well-Being Changed among Adolescents and Medical Students Due to COVID-19 Lockdown in Croatia?. Nutrients 13, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deschasaux-Tanguy M, Druesne-Pecollo N, Esseddik Y, et al. (2020) Diet, physical activity during the COVID-19 lockdown period (March-May 2020): results from the French NutriNet-Sante cohort study. Am J Clin Nutr 113, 924–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Giustino V, Parroco AM, Gennaro A, et al. (2020) Physical activity levels and related energy expenditure during COVID-19 quarantine among the Sicilian active population: a cross-sectional online survey study. Sustainability 12, 4356. [Google Scholar]
- 53. Rogers N, Roberts C, Waterlow N, et al. (2020) Behavioural change towards reduced intensity physical activity is disproportionately prevalent among adults with serious health issues or self-perception of high risk during the UK COVID-19 lockdown. Front Public Health 8, 575091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chopra S, Ranjan P, Singh V, et al. (2020) Impact of COVID-19 on lifestyle-related behaviours-a cross-sectional audit of responses from nine hundred and ninety-five participants from India. Diabetes Metab Syndrome: Clin Res Rev 14, 2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cransac-Miet A, Zeller M, Chagué F, et al. (2021) Impact of COVID-19 lockdown on lifestyle adherence in stay-at-home patients with chronic coronary syndromes: towards a time bomb. Int J Cardiol 323, 285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jia P, Zhang L, Yu W, et al. (2020) Impact of COVID-19 lockdown on activity patterns, weight status among youths in China: the COVID-19 Impact on Lifestyle Change Survey (COINLICS). Int J Obes 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Malkawi SH, Almhdawi K, Jaber AF, et al. (2020) COVID-19 quarantine-related mental health symptoms and their correlates among mothers: a cross sectional study. Matern Child Health J 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Matos DG, Aidar FJ, Almeida-Neto PF, et al. (2020) The impact of measures recommended by the government to limit the spread of coronavirus (COVID-19) on physical activity levels, quality of life, and mental health of Brazilians. Sustainability 12, 9072 [Google Scholar]
- 59. Ruissen MM, Regeer H, Landstra CP, et al. (2021) Increased stress, weight gain, less exercise in relation to glycemic control in people with type 1, type 2 diabetes during the COVID-19 pandemic. BMJ Open Diab Res Care 9, e002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ahmed HO (2020) The impact of social distancing and self-isolation in the last corona COVID-19 outbreak on the body weight in Sulaimani governorate-Kurdistan/Iraq, a prospective case series study. Ann Med Surg 59, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gentile A, Torales J, O’Higgins M, et al. (2020) Phone-based outpatients’ follow-up in mental health centers during the COVID-19 quarantine. Int J Soc Psychiatr 0020764020979732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Romero-Blanco C, Rodríguez-Almagro J, Onieva-Zafra MD, et al. (2020) Sleep Pattern Changes in Nursing Students during the COVID-19 Lockdown. Int J Environ Res Public Health 17, 5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dogas Z, Kalcina LL, Dodig IP, et al. (2020) The effect of COVID-19 lockdown on lifestyle and mood in Croatian general population: a cross-sectional study. Croatian Med J 61, 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Onmez A, Gamsızkan Z, Özdemir Ş, et al. (2020) The effect of COVID-19 lockdown on glycemic control in patients with type 2 diabetes mellitus in Turkey. Diabetes Metab Syndrome: Clin Res Rev 14, 1963–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Karatas S, Yesim T & Beysel S (2021) Impact of lockdown COVID-19 on metabolic control in type 2 diabetes mellitus and healthy people. Primary Care Diabetes (In the Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mason TB, Barrington-Trimis J & Leventhal AM (2021) Eating to cope with the COVID-19 pandemic and body weight change in young adults. J Adolesc Health 68, 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Blaszczyk-Bębenek E, Jagielski P, Boleslawska I, et al. (2020) Nutrition behaviors in Polish adults before, during covid-19 lockdown. Nutrients 12, 3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huber BC, Steffen J, Schlichtiger J, et al. (2020) Altered nutrition behavior during COVID-19 pandemic lockdown in young adults. Eur J Nutr 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Newman E, O’Connor DB & Conner M (2007) Daily hassles and eating behaviour: the role of cortisol reactivity status. Psychoneuroendocrinology 32, 125–132. [DOI] [PubMed] [Google Scholar]
- 70. Jakubowicz D, Barnea M, Wainstein J, et al. (2013) High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 21, 2504–2512. [DOI] [PubMed] [Google Scholar]
- 71. McNeill LH, Kreuter MW & Subramanian SV (2006) Social environment and physical activity: a review of concepts and evidence. Soc Sci Med 63, 1011–1022. [DOI] [PubMed] [Google Scholar]
- 72. Swinburn B, Egger G & Raza F (1999) Dissecting obesogenic environments: the development and application of a framework for identifying and prioritizing environmental interventions for obesity. Prev Med 29, 563–570. [DOI] [PubMed] [Google Scholar]
- 73. Gallè F, Sabella EA, Da Molin G, et al. (2020) Understanding Knowledge and Behaviors Related to CoViD-19 Epidemic in Italian Undergraduate Students: the EPICO Study. Int J Environ Res Public Health 17, 3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Salute M della (2020) Covid-19, how to follow an appropriate and healthy lifestyle when staying at home. http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioNotizieNuovoCoronavirus.jsp?lingua=italiano&menu=notizie&p=dalministero&id=4421 (accessed July 2020).
- 75. Almandoz JP, Xie L, Schellinger JN, et al. (2020) Impact of COVID-19 stay-at-home orders on weight-related behaviours among patients with obesity. Clin Obes 10, e12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kivimäki M, Head J, Ferrie JE, et al. (2006) Work stress, weight gain and weight loss: evidence for bidirectional effects of job strain on body mass index in the Whitehall II study. Int J Obes 30, 982–987. [DOI] [PubMed] [Google Scholar]
- 77. Serlachius A, Hamer M & Wardle J (2007) Stress and weight change in university students in the United Kingdom. Physiol Behav 92, 548–553. [DOI] [PubMed] [Google Scholar]
- 78. Razzoli M & Bartolomucci A (2016) The Dichotomous effect of chronic stress on obesity. Trends Endocrinol Metab 27, 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dallman MF (2010) Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab 21, 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Adam TC & Epel ES (2007) Stress, eating and the reward system. Physiol Behav 91, 449–458. [DOI] [PubMed] [Google Scholar]
- 81. O’Connor DB, Jones F, Conner M, et al. (2008) Effects of daily hassles and eating style on eating behavior. Health Psychol 27, S20–S31. [DOI] [PubMed] [Google Scholar]
- 82. Heatherton TF & Baumeister RF (1991) Binge eating as escape from self-awareness. Psychol Bull 110, 86–108. [DOI] [PubMed] [Google Scholar]
- 83. Haddad C, Zakhour M, Bou Kheir M, et al. (2020) Association between eating behavior, quarantine/confinement stressors during the Coronavirus disease 2019 outbreak. J Eat Disord 8, 40. doi: 10.1186/s40337-020-00317-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Khan MA, Shah SM, Shehab A, et al. (2019) Screen time and metabolic syndrome among expatriate adolescents in the United Arab Emirates. Diabetes Metab Syndrome: Clin Res Rev 13, 2565–2569. [DOI] [PubMed] [Google Scholar]
- 85. Stults-Kolehmainen MA & Sinha R (2014) The effects of stress on physical activity and exercise. Sports Med 44, 81–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Seigel K, Broman J-E & Hetta J (2002) Behavioral activation or inhibition during emotional stress-implications for exercise habits and emotional problems among young females. Nord J Psychiatry 56, 441–446. [DOI] [PubMed] [Google Scholar]
- 87. Vlahov D, Galea S, Resnick H, et al. (2002) Increased use of cigarettes, alcohol, and marijuana among Manhattan, New York, residents after the September 11th terrorist attacks. Am J Epidemiol 155, 988–996. [DOI] [PubMed] [Google Scholar]
- 88. Chiolero A, Faeh D, Paccaud F, et al. (2008) Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr 87, 801–809. [DOI] [PubMed] [Google Scholar]
- 89. Poelman MP, Gillebaart M, Schlinkert C, et al. (2020) Eating behavior and food purchases during the COVID-19 lockdown: a cross-sectional study among adults in the Netherlands. Appetite 157, 105002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ruiz-Roso MB, de Carvalho Padilha P, Mantilla-Escalante DC, et al. (2020) Covid-19 confinement, changes of adolescent’s dietary trends in Italy, Spain, Chile, Colombia, Brazil. Nutrients 12, 1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ferrer R & Klein WM (2015) Risk perceptions and health behavior. Curr Opin Psychol 5, 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Raude J, Lecrique JM, Lasbeur L, et al. (2020) Determinants of preventive behaviors in response to the COVID-19 pandemic in France: comparing the sociocultural, psychosocial and social cognitive explanations. Front Psychol 11, 584500. doi: 10.3389/fpsyg.2020.584500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Adams EL, Caccavale LJ, Smith D, et al. (2020) Food insecurity, the home food environment, and parent feeding practices in the era of COVID-19. Obesity 28, 2056–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Micha R, Mannar V, Afshin A, et al. (2020) 2020 Global nutrition report: action on equity to end malnutrition. Development Initiatives. https://eprints.mdx.ac.uk/id/eprint/30645
- 95. UNSCN (2020) COVID-19 pandemic: the evolving impact on how people meet the food system. https://www.unscn.org/en/news-events/recent-news?idnews=2065 (accessed January 2021).
- 96. WHO (2020) As more go hungry and malnutrition persists, achieving Zero Hunger by 2030 in doubt, UN report warns. https://www.who.int/news/item/13–07–2020-as-more-go-hungry-and-malnutrition-persists-achieving-zero-hunger-by-2030-in-doubt-un-report-warns (accessed February 2021).
- 97. United Nations World Food Programme (2020) WFP at a glance. https://www.wfp.org/stories/wfp-glance (accessed December 2020).
- 98. Paslakis G, Dimitropoulos G & Katzman DK (2021) A call to action to address COVID-19–induced global food insecurity to prevent hunger, malnutrition, and eating pathology. Nutr Rev 79, 114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schanzenbach D & Pitts A (2020) How much has food insecurity risen? Evidence from the Census Household Pulse Survey. Institute for Policy Research (IPR) Rapid Research Report. Northwestern Institute for Policy Research. https://www.ipr.northwestern.edu/news/2020/schanzenbach-household-pulse-survey-analysis.html
- 100. El Zein A, Colby SE, Zhou W, et al. (2020) Food insecurity is associated with increased risk of obesity in US college students. Curr Dev Nutr 4, nzaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sheth J (2020) Impact of Covid-19 on consumer behavior: will the old habits return or die? J Bus Res 117, 280–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mehta S, Saxena T & Purohit N (2020) The new consumer behaviour paradigm amid COVID-19: permanent or transient? J Health Manag 22, 291–301. [Google Scholar]
- 103. Baraniuk C (2020) Fears grow of nutritional crisis in lockdown UK. BMJ 370, m3193. [DOI] [PubMed] [Google Scholar]
- 104. Hajifathalian K, Kumar S, Newberry C, et al. (2020) Obesity is associated with worse outcomes in COVID-19: analysis of Early Data From New York City. Obesity 28, 1606–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ho FK, Celis-Morales CA, Gray SR, et al. (2020) Modifiable, non-modifiable risk factors for COVID-19: results from UK Biobank prospective cohort study. BMJ Open 10, e040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Huang Y, Yao LU, Huang Y-M, et al. (2020) Obesity in patients with COVID-19: a systematic review, meta-analysis. Metabolism 154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hill MA, Sowers JR & Mantzoros CS (2020) Commentary: COVID-19 and obesity pandemics converge into a syndemic requiring urgent and multidisciplinary action. Metabolism 114, 154408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Eknoyan G (2006) A history of obesity, or how what was good became ugly and then bad. Adv Chronic Kidney Dis 13, 421–427. [DOI] [PubMed] [Google Scholar]
- 109. Gagnier JJ, Moher D, Boon H, et al. (2012) Investigating clinical heterogeneity in systematic reviews: a methodologic review of guidance in the literature. BMC Med Res Method 12, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114521000921.
click here to view supplementary material


