Abstract
The goal of this study was to examine: 1) differences in parent-reported prosocial and antisocial behaviors between children and adolescents with and without prenatal alcohol exposure (PAE); 2) differences in gray matter volumes of brain areas supporting social cognition between children and adolescents with and without PAE; 3) correlations between gray matter volumes of brain areas supporting social cognition and parent-reported prosocial and antisocial behaviors. Parents of children and adolescents ages 8–16 years completed measures on their prosocial and antisocial behaviors (i.e., Behavior Assessment Scale for Children, Vineland Adaptive Behaviors Scales, and Child Behavior Checklist) (n = 84; 41 with PAE, 43 without PAE). Seventy-nine participants (40 with PAE, 39 without PAE) also completed a structural Magnetic Resonance Imaging (MRI) scan with quality data. Gray matter volumes of seven brain areas supporting social cognitive processes were computed using automated procedures (FreeSurfer 6.0): bilateral fusiform gyrus, superior temporal gyrus, medial orbitofrontal cortex, lateral orbitofrontal cortex, posterior cingulate cortex, precuneus, and temporal pole.
Children and adolescents with PAE showed decreased prosocial behaviors and increased antisocial behaviors as well as smaller volumes of the precuneus and lateral orbitofrontal cortex, even when controlling for total intracranial volume. Social brain volumes were not significantly correlated with prosocial or antisocial behaviors.
These findings suggest that children and adolescents with PAE show worse social functioning and smaller volumes of brain areas supporting self-awareness, perspective-taking and emotion-regulation than their same-age peers without PAE.
Keywords: Fetal Alcohol Spectrum Disorder, Adolescents, Social Behaviors, Gray Matter Volume
Introduction
Prenatal alcohol exposure (PAE) is a leading cause of neurodevelopmental impairment in children and adolescence, with prevalence rates indicating it may affect up to five out of every one hundred individuals [1]. PAE is linked to a variety of developmental delays which can present as cognitive, behavioral, and/or adaptive functioning deficits. Children and adolescents with PAE often present with social difficulties [2–4]. These include poor boundaries with strangers [5], difficulty interpreting social cues [6,7], diminished capacity to inhibit inappropriate behaviors [7], poor perspective-taking ability [8], trouble maintaining social relationships [9], and aggressive behaviors [10]. Social difficulties in children and adolescents with PAE tend to become more pronounced with age [11,12] and are associated with a variety of poor outcomes, including school dropout and delinquency, and anxiety and depression [13,14]. Understanding the neurodevelopmental abnormalities that are associated with these social impairments in children and adolescents with PAE is therefore critical to inform intervention programs designed to prevent these poor outcomes.
Adolescence is a period of increased orientation towards peers and significant development of social skills, accompanied by marked structural and functional changes in the “social brain”, or brain areas involved in social cognitive processes [15–17]. Basic social cognitive processes, such as processing of faces, eye gaze, and motion, are subserved by the fusiform gyrus, superior temporal gyrus (STG) and amygdala [15–20]. Higher-order social cognitive processes, such as self-referential processing (i.e., self-awareness) and perspective-taking, are supported by the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), and precuneus [15–20]. The lateral orbitofrontal cortex (lOFC) is involved in emotion-regulation, while the temporal pole is involved in applying social knowledge (e.g., social scripts, or expected behaviors in specific social situations) [17].
Children and adolescents with social deficits, such as those with Autism Spectrum Disorder (ASD), have altered gray matter volumes in these social brain areas; both larger and smaller volumes of different areas compared to typically developing children and adolescents have been reported [21]. Gray matter volumes in these social brain areas are further correlated with social skills deficits in children and adolescents with ASD [22]. Only a handful of studies have examined gray matter volume differences in social brain areas in children and adolescents with PAE, with mixed findings. We observed no significant differences in amygdala volumes between children and adolescents with PAE and their same-age peers without PAE [23], consistent with others [24]. In addition, Gautam and colleagues [24] did not observe significant differences in PCC or temporal pole volumes between children and adolescents with PAE and those without PAE. In contrast, three other studies found that children and adolescents with PAE had smaller amygdala volumes than their same-age peers without PAE [25–27]. Nevertheless, little is known about gray matter volumes across the rest of the “social brain” including both subcortical and cortical areas, and how these brain volumes correlate with social skills in children with and without PAE. Understanding the neural mechanisms underlying social difficulties in children and adolescents with PAE may help inform intervention programs designed to improve these social difficulties.
The aims of the present study were to examine whether: 1) children and adolescents (ages 8–16 years) with PAE show lower levels of parent-reported prosocial behaviors and higher levels of antisocial behaviors compared to their same-age peers without PAE [2–4]; 2) children and adolescents (aged 8–16 years) with PAE have smaller gray matter volumes of brain areas supporting social cognition (i.e., fusiform gyrus, STG, mOFC, lOFC, PCC, precuneus, temporal pole) than their same-age peers without PAE [25–29]; 3) gray matter volumes of brain areas supporting social cognition are correlated with parent-reported prosocial and antisocial behaviors [22].
Results
Parent-reported prosocial and antisocial behaviors
The repeated-measures ANOVA revealed a significant effect of group on parent-reported prosocial and antisocial behaviors (F(1,82) = 4.20; p = 0.044; partial η2 = 0.049) (see Figure 1). Parameter estimates showed that parents of children with PAE reported significantly lower levels of prosocial behaviors on the BASC Social Skills scale and Vineland Socialization Domain compared to parents of control participants (see Table 2). Parents of children with PAE further reported significantly more social problems on the CBCL and more antisocial behaviors on the BASC Bullying scale compared to parents of control participants (see Table 2).
Figure 1.
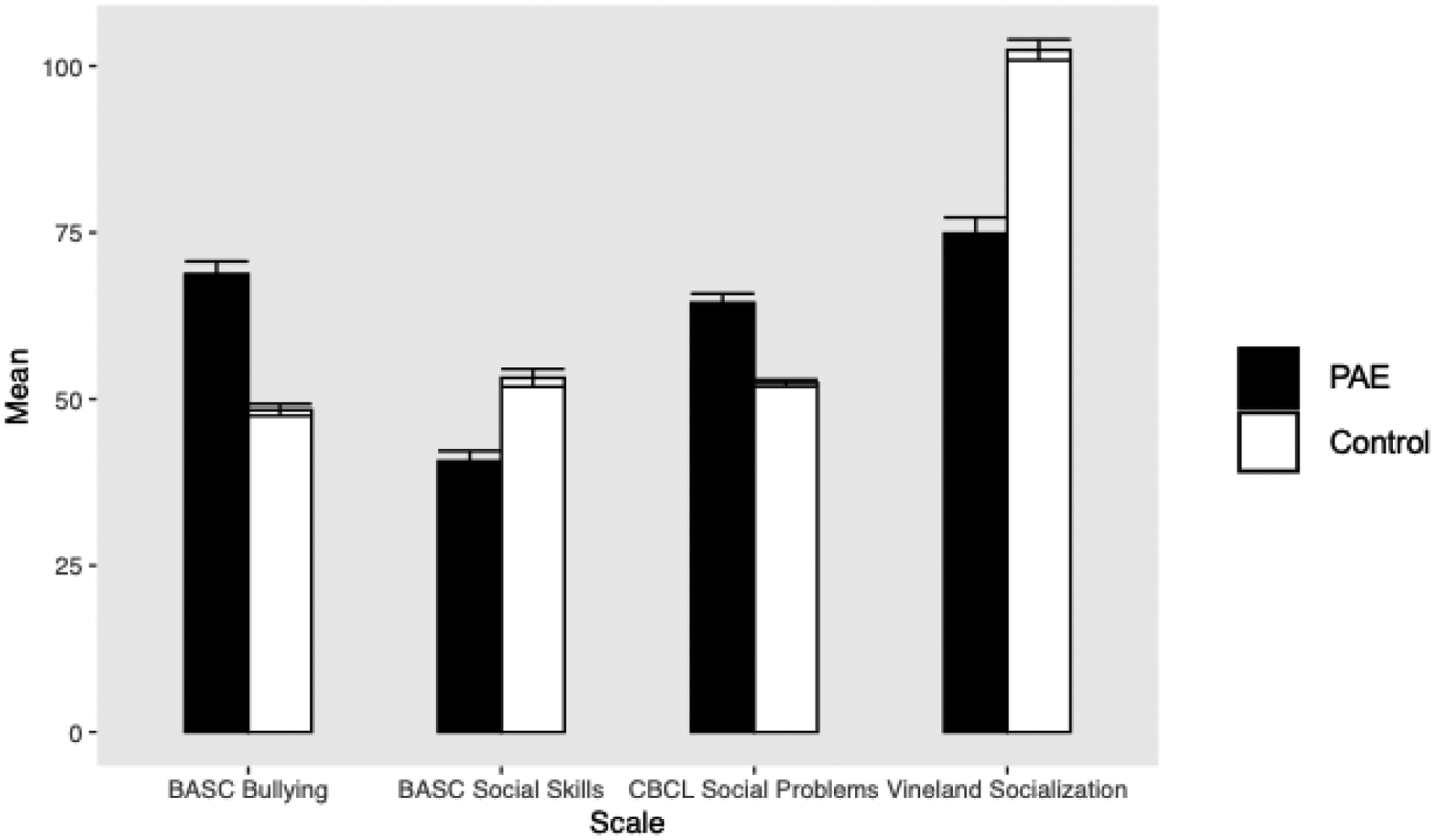
Differences in parent-reported social behaviors between children and adolescents with prenatal alcohol exposure (PAE; n = 41) and control participants without PAE (n = 43)
Note. Error bars represent standard errors of the mean.
BASC = Behavior Assessment System for Children; CBCL = Child Behavior Checklist
Table 2.
Comparison of parent-report measures on pro- and anti-social behavior between participants with prenatal alcohol exposure (PAE; n = 41) and control participants (n=43)
| Measures | Mean | SD | t | p | ||
|---|---|---|---|---|---|---|
| PAE | Control | PAE | Control | |||
| BASC Social Skills | 41.29 | 53.05 | 9.78 | 9.21 | −5.718 | <0.001 |
| BASC Bullying | 68.05 | 48.12 | 12.70 | 5.95 | 9.282 | <0.001 |
| CBCL Social Problems | 64.15 | 52.40 | 8.99 | 3.33 | 8.019 | <0.001 |
| Vineland Socialization | 74.85 | 102.42 | 15.40 | 10.24 | −9.702 | <0.001 |
Note. SD = standard deviation; CBCL = Child Behavior Checklist; BASC = Behaviors Assessment Scale for Children.
Group differences in gray matter volumes of social brain areas
When total intracranial volume was not controlled for, a significant effect of group on the volumes of social brain regions was observed (F(1,77) = 10.02; p = 0.002; partial η2 = 0.115). Children with PAE has significantly smaller volumes of the fusiform gyrus, lateral OFC, PCC, and precuneus (see Table 3).
Table 3.
Comparison of social brain region volumes (mm3) between participants with prenatal alcohol exposure (PAE; n = 40) and control participants (n = 39)
| Region | Mean | SD | t unc * | p unc * | t corr ** | p corr ** | ||
|---|---|---|---|---|---|---|---|---|
| PAE | Control | PAE | Control | |||||
| Fusiform Gyrus | 10995 | 11820 | 1398 | 1417 | −2.604 | 0.011 | −1.137 | 0.259 |
| Lateral OFC | 8895 | 9545 | 864 | 918 | −3.236 | 0.002 | −2.065 | 0.042 |
| Medial OFC | 6094 | 6150 | 790 | 831 | −0.307 | 0.759 | 0.763 | 0.448 |
| PCC | 3974 | 4273 | 470 | 522 | −2.670 | 0.009 | −1.659 | 0.101 |
| Precuneus | 12561 | 13961 | 1765 | 1790 | −3.498 | 0.001 | −2.453 | 0.016 |
| STG | 14935 | 15641 | 1557 | 1770 | −1.883 | 0.063 | −0.468 | 0.641 |
| Temporal Pole | 2503 | 2618 | 249 | 271 | −1.966 | 0.053 | −1.198 | 0.234 |
Note. SD = standard deviation; OFC = orbitofrontal cortex; PCC = posterior cingulate cortex; STG= Superior Temporal Gyrus.
uncorrected for total intracranial volume
corrected for total intracranial volume.
When we controlled for total intracranial volume, the effect of group on the volumes of social brain regions became significant at a trend level (F(1,76) = 3.58; p = 0.062; partial η2 = 0.045). However, parameter estimates showed that children with PAE still had significantly smaller volumes of the lateral OFC and precuneus (see Table 3 and Figure 2), even after controlling for total intracranial volume.
Figure 2.
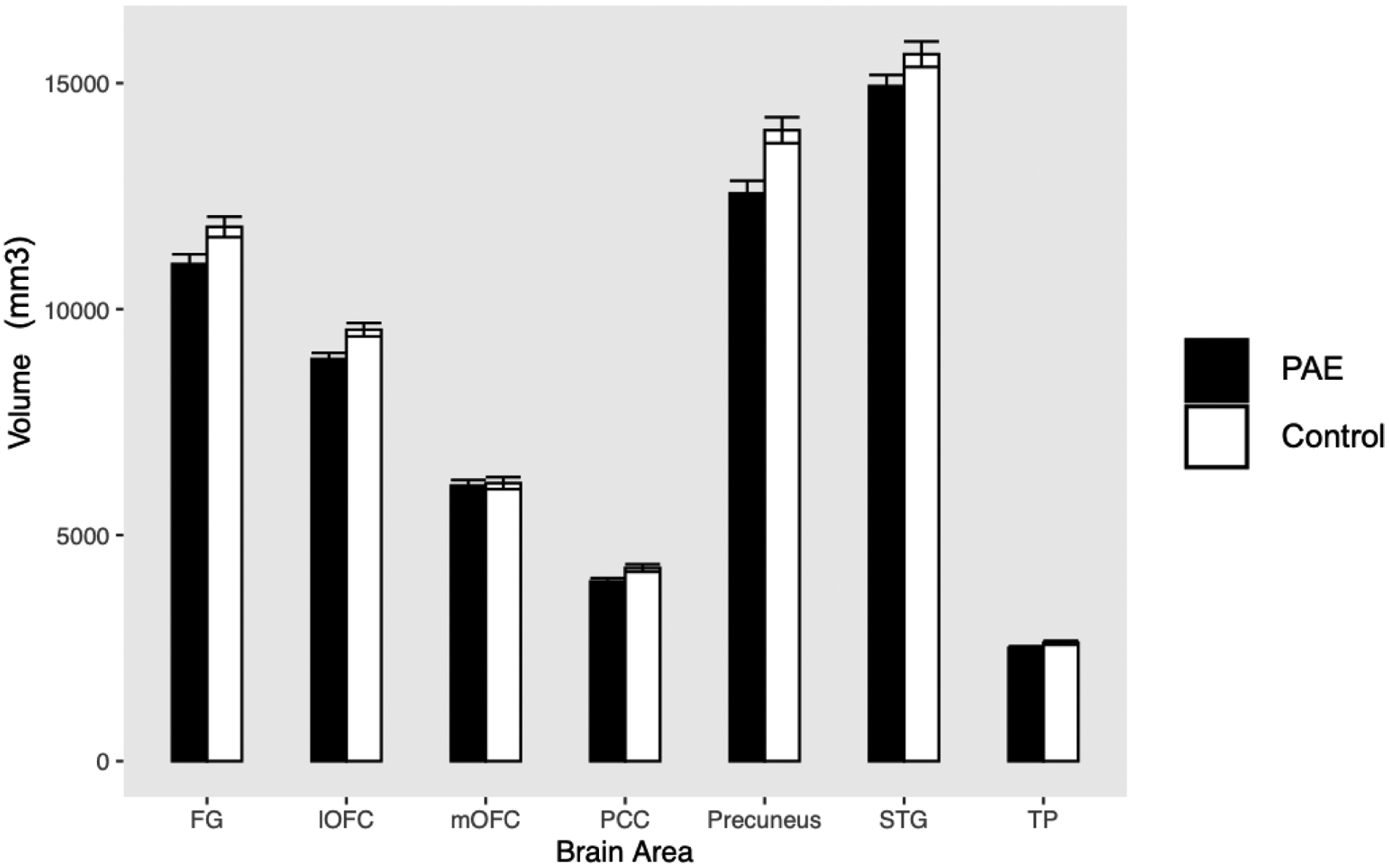
Differences in gray matter volumes between children and adolescents with prenatal alcohol exposure (PAE; n = 40) and control participants without PAE (n = 39)
Note. Error bars represent standard errors of the mean.
FG = fusiform gyrus; lOFC = lateral orbitofrontal cortex; mOFC = medial orbitofrontal cortex; PCC = posterior cingulate cortex; STG = superior temporal gyrus; TP = temporal pole
Correlations between gray matter volumes and prosocial and antisocial behaviors
No significant correlations between brain volumes and prosocial and antisocial behaviors were observed, neither when controlling for total intracranial volume (all p’s >0.26) nor without controlling for this variable (all p’s >0.09).
Discussion
The aims of the present study were to examine whether: 1) children and adolescents with PAE differ from those without PAE in their parent-reported prosocial and antisocial behaviors; 2) children and adolescents with PAE differ from those without PAE in terms of gray matter volumes of brain areas supporting social cognition (i.e., fusiform gyrus, STG, mOFC, lOFC, PCC, precuneus, temporal pole); 3) gray matter volumes of brain areas supporting social cognition are correlated with parent-reported prosocial and antisocial behaviors in children and adolescents with and without PAE. We found that children and adolescents with PAE showed lower levels of parent-reported prosocial behaviors and higher levels of antisocial behaviors and social problems compared to those without PAE. Children and adolescents with PAE further had smaller gray matter volumes of the precuneus and lOFC than those without PAE, even after controlling for total intracranial volumes. Gray matter volumes were not significantly correlated with parent-reported prosocial and antisocial behaviors.
The findings of this study are consistent with prior studies showing lower levels of parent reported social skills [2–4] and smaller brain volumes across multiple brain areas in children and adolescents with PAE [25–29]. Consistent with a recent study focusing on social brain volumes in children and adolescents with PAE [24], we did not observe smaller volumes of the PCC and temporal pole in these children and adolescents. Instead, we found that the precuneus and lOFC were smaller in children and adolescents with PAE compared to those without PAE. The precuneus is involved in self-referential processing (i.e., self-awareness) and perspective-taking, while the lOFC contributes to emotion-regulation [15–20]. Gray matter volumes of the precuneus and lOFC have been linked to social skills, including perspective-taking, in prior studies [30, 31]. Future research should examine whether social cognitive processes (e.g., perspective-taking, emotion-regulation) mediate the associations between precuneus and lOFC volumes and prosocial and antisocial behaviors in children and adolescents with PAE.
While children and adolescents with PAE had smaller precuneus and lOFC volumes, these volumes were not significantly associated with parent-reported prosocial and antisocial behaviors. Volumes of social brain areas may be more strongly correlated with measures of the social cognitive processes they subserve (e.g., standardized measures of perspective-taking, affect recognition or inhibition of emotional stimuli) than with broad social behaviors. Future studies could also investigate how brain volumes are related to altered functional and structural connectivity of the brain and whether these network measures may better predict social functioning.
Limitations of the current study include the use of parent-report measures of social functioning and the cross-sectional design of the study. The parent-report measures we used in the present study have been shown to have high reliability and validity [32–34] and we observed high correlations (r’s up to 0.84) between the different measures in this study - suggesting some degree of coherent construct validity. It is important to note that parents may be less aware of their child’s social functioning in settings other than the home, such as in school or when interacting with peers outside of school and the home (e.g., the mall or other friend’s homes). Future studies could therefore include teacher-report and/or peer-report measures of social functioning in addition to parent-report measures. Longitudinal follow-up studies could evaluate whether social brain volumes predict social behaviors at a later time point, and whether changes in brain volumes are associated with changes in social behaviors.
Conclusions
To conclude, we found that children and adolescents with PAE showed fewer parent-reported prosocial behaviors and more antisocial behaviors, accompanied by smaller volumes of brain areas supporting self-referential processing, perspective-taking and emotion-regulation (i.e., precuneus and lOFC). Future studies should explore whether social cognitive processes supported by the precuneus and lOFC (i.e., self-referential processing, perspective-taking and emotion-regulation) mediate associations between the volumes of these brain areas and prosocial and antisocial behaviors, and whether social skills interventions targeting these social cognitive processes could improve social outcomes in children with PAE.
Methods and materials
Participants and Procedures
Children and adolescents ages 8–16 years were included in the present study (see Table 1 for demographic information). All participants completed cognitive testing and a physical examination conducted by a highly experienced dysmorphologist (KLJ). Parents of eighty-four children and adolescents (41 with PAE, 43 without PAE) completed parent-report measures in regard to their child’s social behavior. Eighty-three participants (n = 44 with PAE, n = 39 control participants) completed a structural MRI (i.e., T1-weighted) scan, but MRI images of 4 participants with PAE had to be excluded due to visible processing errors (see below), resulting in a total of 79 participants (40 with PAE, 39 control participants) included in MRI analyses. All procedures were carried out after the parent/legal guardian had provided written informed consent and the child had assented to the study proceedings. All procedures were approved by the University of Minnesota’s Institutional Review Board (IRB) and all participants were compensated for their time and reimbursed for any travel expense.
Table 1.
Characteristics of the participants
| N(%) or mean (SD) | PAE (n=41) | Control (n=43) | p |
|---|---|---|---|
| Age | 11.54 (2.35) | 11.95 (2.62) | 0.45 |
| Gender | |||
| Male | 20 (48.8%) | 23 (53.5%) | 0.67 |
| Female | 21 (51.2%) | 20 (46.5%) | |
| Race | |||
| White | 18 (43.9%) | 42 (97.7%) | <0.001 |
| Black or African American | 4 (9.8%) | 0 (0 %) | |
| American Indian/Alaska Native | 2 (4.9%) | 0 (0%) | |
| Asian | 2 (4.9%) | 0 (0 %) | |
| Native Hawaiian or Other Pacific Islander | 1 (2.4%) | 0 (0 %) | |
| More than One Race | 14 (34.1%) | 1 (2.3%) | |
| FSIQ | 92 (14.76) | 115 (12.32) | <0.001 |
| Alcohol Exposure | |||
| Confirmed | 37 (90.2%) | ||
| Suspected | 4 (9.8%) | ||
| Other Drug Exposure | |||
| None | 7 (17.1%) | ||
| Confirmed | 21 (51.2%) | ||
| Suspected | 13 (31.7%) | ||
| Dysmorphic Facial Features | |||
| Lip (score 4 or 5) | 10 (24.4%) | 3 (7.0%) | 0.13 |
| Philtrum (score 4 or 5) | 15 (36.6%) | 3 (7.0%) | 0.01 |
| Palpebral Fissure (≤10th percentile) | 4 (9.8%) | 3 (7.0%) | 0.95 |
| ≥ 2 Facial Features Present | 12 (30.0%) | 1 (2.3%) | 0.001 |
| Growth Deficiency (≤10thpercentile) | |||
| Height | 5 (12.2%) | 0 (0.0%) | 0.05 |
| Weight | 2 (4.9%) | 3 (7.0%) | 0.39 |
| Deficient Brain Growth (≤10thpercentile) | |||
| Occipital-Frontal Circumference (OFC) | 4 (9.8%) | 0 (0.0%) | 0.08 |
| IOM Diagnostic Category | |||
| FAS | 2 (4.9%) | ||
| Partial FAS | 11 (26.8%) | ||
| ARND | 28 (68.3%) | ||
Note: Six participants in the PAE group and 11 participants in the control group did not have available physical exam information. The four participants with suspected alcohol exposure were included for the following reasons: two met criteria for pFAS, one had adoption records indicating maternal alcohol use, and the final participant had a biological sibling with an FASD diagnosis along with record of the biological mother’s alcohol abuse.
All participants enrolled in this study were part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). Participants within the PAE group were recruited from the University of Minnesota’s Fetal Alcohol Spectrum Disorder (FASD) Clinic between the years of 2017 and 2020. Prenatal alcohol exposure was determined through maternal report, adoption, legal, and/or medical records. Participants were included in the PAE group if there was evidence of >13 drinks per week or >4 drinks per occasion at least once per week during pregnancy or if similar amounts were suspected in an individual with a predetermined FASD diagnosis. When detailed information about the amount of alcohol use was not available, inclusion in this group was determined based on the available documentation that indicated clear FASD diagnostic criteria (i.e., medical records, adoption records, and physical examinations). More detail in regard to the documentation utilized can be found in Table 1.
Participants in the Control group (i.e., without PAE) were recruited through mailings, online advertisements, and referrals. Individuals were excluded if they were prenatally exposed to alcohol or other drugs (aside from tobacco and caffeine). Participants were included in the control group if there was only minimal exposure during pregnancy (<1 drink per week, never >2 drinks per occasion). Only 5 control participants had minimal exposure (i.e., a total of 1–3 drinks throughout the entire pregnancy). When we excluded these participants from the analyses, our findings were unchanged. Participants were excluded from the control group if they were ever diagnosed with a psychiatric disorder. The same was not true of the PAE group, as internalizing and externalizing disorders are common among children prenatally exposed to alcohol [35].
Exclusion criteria that applied to both groups included the presence of a developmental disorder other than FASD, a birthweight below 1500 grams, participant substance abuse, ineligibility for MRI scanning (i.e., any implanted metal devices, dental hardware, and/or claustrophobia), neurological conditions, traumatic brain injury, or a psychiatric disorder that would hinder participation (i.e., psychosis).
Prosocial and Antisocial Behaviors
Parent-reported social behaviors were measured using the Child Behavior Checklist (CBCL [32]), the Behavior Assessment System for Children Third Edition (BASC-3 [33]), and the Vineland Adaptive Behavior Scales Third Edition (Vineland-3 [34]). All three assessments are parent report questionnaires that were completed by a parent/legal guardian in regard to their child’s behavior.
The CBCL [32] reports on a child’s behavior within the last six months. Questions are rated on a Likert-scale (0 = not true, 1 = somewhat/sometimes true, 2 = very true/often true) and yield T-scores (M = 50, SD = 10). The Social Problems scale was used as an outcome measure (higher scores indicate more severe social problems), which has been shown to have high reliability and validity [32].
The BASC-3 [33] also reports on a child’s behavior within the last few months and utilizes a Likert-scale format (1 = Never, 2 = Sometimes, 3 = Often, 4 = Almost Always). The main clinical scales and content scales are reported as T-scores (M = 50, SD = 10). We included the Social Skills Clinical Scale (higher T-scores indicate greater prosocial behaviors) and the Bullying Content Scale (higher T-scores indicate more frequent participation in bullying behaviors) in our analyses. Both BASC-3 scales have high reliability and validity [33].
The Vineland-3 [34] assesses a child’s current adaptive functioning ability across a variety of domains. The Parent/Caregiver form is structured on a 3-point Likert Scale (0 = Never, 1 = Sometimes, 2 = Usually or often) and provides standard scores for the adaptive behavior domains (M = 100, SD = 15). The Vineland Socialization domain was utilized in analyses, with higher scores indicating higher social functioning. The Vineland has been shown to have high reliability and validity [34].
MRI Acquisition and Processing
For all subjects, MRI data were acquired at the University of Minnesota’s Center for Magnetic Resonance Research on one of two 3T Siemens Prisma scanners (Siemens, Erlangen, Germany) equipped with standard 32-channel head coils. T1-weighted (multi-echo MP-RAGE TR = 2500 ms; TEs = 1.8, 3.6, 5.4, 7.2 ms; 208 slices, voxel size = 0.8 mm isotropic, FOV = 256 × 240 mm, flip angle = 8 degrees) and T2-weighted (SPACE TR = 3200 ms, TE = 564 ms, 208 slices, voxel size = 0.8 mm isotropic, FOV = 256 × 240 mm, variable flip angle) images were collected. The Human Connectome Project T1- and T2- weighted sequences, which include volumetric navigators in the sequences that allow for prospective motion correction and reacquisition k-space that suffer from subject motion, were used in this study [36]. The Human Connectome Project Minimal Preprocessing Pipeline (v4.0.1) was used to preprocess the structural data [37]. This pipeline aligned T1-weighted and T2-weighted images, performed bias field and gradient distortion corrections, and registered the data to MNI space before proceeding with FreeSurfer (v6.0.0) cortical reconstruction (surfer.nmr.mgh.harvard.edu [38]). FreeSurfer processing included removal of skull and other non-brain tissue, automated Talairach transformation, intensity normalization, segmentation, tessellation of the grey matter / white matter boundary, topology correction, surface deformation, refinement of the pial surface based on the T2-weighted image, and cortical parcellation using the Desikan-Killiany atlas [39]. Volumes of FreeSurfer-defined cortical parcels, as well as estimates of total intracranial volume, were used in the subsequent analyses. To increase the reproducibility of our findings, we encapsulated our preprocessing pipeline using Singularity [40]. This container will be made available upon request, by contacting the corresponding author.
A trained operator (DJR) visually inspected the FreeSurfer parcellations using the tools available from the ENIGMA imaging protocol (http://enigma.ini.usc.edu [41]). Parcel volumes were plotted and outliers were flagged for closer inspection. Data were excluded from the analysis if visible processing errors (such as failed boundary identification) were present (n = 4 with PAE), resulting in a total of 79 participants (40 with PAE, 39 control participants) included in MRI analyses.
Gray matter volumes – which reflect both cortical thickness and surface area - for the following “social brain” areas were included in the analyses: fusiform gyrus, lateral OFC, medial OFC, PCC, precuneus, STG, and temporal pole. We focused on amygdala volumes and correlations with internalizing behaviors in children and adolescents with and without PAE in a previous study using the same participants [23], which is why we did not include amygdala volumes in the analyses we report on here. Given that we had no a priori hypotheses about lateralized effects, we computed bilateral volumes by averaging the right and left hemisphere volumes for each brain area and used these bilateral volumes in all analyses.
Data Analyses
SPSS (IBM SPSS Statistics for Macintosh, Version 25.0) was used for all analyses. To account for the correlations among the different social behavior scales (r’s up to 0.84) and among the brain volumes (r’s up to 0.67) and to reduce the number of univariate comparisons, group differences in social behaviors and social brain volumes were evaluated by performing a repeated-measures ANOVA and an ANCOVA, respectively. The repeated-measures ANOVA included the four social behavior scales (i.e., CBCL Social Problems, Vineland Socialization Domain, BASC Social Skills, BASC Bullying) as within-subjects variables and group (PAE vs. Controls) as the between-subjects factor. The repeated-measures ANCOVA included the seven social brain volumes (i.e., fusiform gyrus, lateral OFC, medial OFC, PCC, precuneus, STG, temporal pole) as within-subjects variables, group (PAE vs. Controls) as the between-subjects factor and total intracranial volume as a covariate. Total intracranial volume was included as a covariate in order to account for the group difference (Controls > PAE) in intracranial volume (t = −248, p = 0.015), and the significant correlations between intracranial volume and regional brain volumes (r’s 0.26–0.75; p’s <0.02). We also performed this analysis without covarying for intracranial volume.
Finally, partial correlations (controlling for total intracranial volume) between brain volumes and the social behavior scales were computed within each group separately. Only volumes of brain areas that were significantly different between the two groups were included in these correlation analyses. Given that these correlations were computed within each group and were thus not affected by group differences in intracranial volume, we also computed correlations without controlling for total intracranial volume.
Children and adolescents with prenatal alcohol exposure (PAE) showed poor social functioning
Children and adolescents with PAE had smaller gray matter volumes of the precuneus and lateral orbitofrontal cortex than controls
Atypical gray matter volumes of brain areas involved in perspective-taking and emotion-regulation may contribute to the social deficits in children and adolescents with PAE
Sources of Support:
All of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD; www.cifasd.org), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
Support for this research was provided by the NIAAA (5U01AA026102, 5U01AA014834, 5U24AA014815, 5U24AA014811, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Biotechnology Research Center (P41 EB015 894), the NINDS Institutional Center Core Grants to Support Neuroscience Research (P30 NS076408), and the High Performance Connectome Upgrade for Human 3T MR Scanner (1S10OD017974-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare that there are no real or perceived conflicts of interest.
References
- [1].May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental Disabilities Research Reviews 2009;15:176–92. 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- [2].Jirikowic T, Kartin D, Olson HC. Children with Fetal Alcohol Spectrum Disorders: A Descriptive Profile of Adaptive Function. Can J Occup Ther 2008;75:238–48. 10.1177/000841740807500411. [DOI] [PubMed] [Google Scholar]
- [3].Kully-Martens K, Denys K, Treit S, Tamana S, Rasmussen C. A Review of Social Skills Deficits in Individuals with Fetal Alcohol Spectrum Disorders and Prenatal Alcohol Exposure: Profiles, Mechanisms, and Interventions. Alcoholism: Clinical and Experimental Research 2012;36:568–76. 10.1111/j.1530-0277.2011.01661.x. [DOI] [PubMed] [Google Scholar]
- [4].Ware AL, Glass L, Crocker N, Deweese BN, Coles CD, Kable JA, et al. Effects of Prenatal Alcohol Exposure and Attention-Deficit/Hyperactivity Disorder on Adaptive Functioning. Alcoholism: Clinical and Experimental Research 2014;38:1439–47. 10.1111/acer.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nanson JL. Autism in Fetal Alcohol Syndrome: A Report of Six Cases. Alcoholism: Clinical and Experimental Research 1992;16:558–65. 10.1111/j.1530-0277.1992.tb01417.x. [DOI] [PubMed] [Google Scholar]
- [6].Carmichael Olson H, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological Deficits in Adolescents with Fetal Alcohol Syndrome: Clinical Findings. Alcoholism: Clinical and Experimental Research 1998;22:1998–2012. 10.1111/j.1530-0277.1998.tb05909.x. [DOI] [PubMed] [Google Scholar]
- [7].Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal Alcohol Syndrome in Adolescents and Adults. JAMA 1991;265:1961–7. 10.1001/jama.1991.03460150065025. [DOI] [PubMed] [Google Scholar]
- [8].Stevens SA, Dudek J, Nash K, Koren G, Rovet J. Social Perspective Taking and Empathy in Children with Fetal Alcohol Spectrum Disorders. J Int Neuropsychol Soc 2015;21:74–84. 10.1017/S1355617714001088. [DOI] [PubMed] [Google Scholar]
- [9].Bishop S, Gahagan S, Lord C. Re-examining the core features of autism: a comparison of autism spectrum disorder and fetal alcohol spectrum disorder. Journal of Child Psychology and Psychiatry 2007;48:1111–21. 10.1111/j.1469-7610.2007.01782.x. [DOI] [PubMed] [Google Scholar]
- [10].Tsang TW, Lucas BR, Olson HC, Pinto RZ, Elliott EJ. Prenatal Alcohol Exposure, FASD, and Child Behavior: A Meta-analysis. Pediatrics 2016;137. 10.1542/peds.2015-2542. [DOI] [PubMed] [Google Scholar]
- [11].Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of Social Abilities of Children with Fetal Alcohol Syndrome to Those of Children with Similar IQ Scores and Normal Controls. Alcoholism: Clinical and Experimental Research 1998;22:528–33. 10.1111/j.1530-0277.1998.tb03684.x. [DOI] [PubMed] [Google Scholar]
- [12].Whaley SE, O’Connor MJ, Gunderson B. Comparison of the Adaptive Functioning of Children Prenatally Exposed to Alcohol to a Nonexposed Clinical Sample. Alcoholism: Clinical and Experimental Research 2001;25:1018–24. 10.1111/j.1530-0277.2001.tb02311.x. [DOI] [PubMed] [Google Scholar]
- [13].Paetsch JJ, Bertrand LD. The Relationship Between Peer, Social, and School Factors, and Delinquency Among Youth. Journal of School Health 1997;67:27–32. 10.1111/j.1746-1561.1997.tb06291.x. [DOI] [PubMed] [Google Scholar]
- [14].Patterson GR, Forgatch MS, Yoerger KL, Stoolmiller M. Variables that initiate and maintain an early-onset trajectory for juvenile offending. Dev Psychopathol 1998;10:531–47. 10.1017/s0954579498001734. [DOI] [PubMed] [Google Scholar]
- [15].Blakemore S-J. Development of the Social Brain during Adolescence. Quarterly Journal of Experimental Psychology 2008;61:40–9. 10.1080/17470210701508715. [DOI] [PubMed] [Google Scholar]
- [16].Blakemore S-J, Mills KL. Is Adolescence a Sensitive Period for Sociocultural Processing? Annual Review of Psychology 2014;65:187–207. 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- [17].Burnett S, Sebastian C, Cohen Kadosh K, Blakemore S-J. The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience & Biobehavioral Reviews 2011;35:1654–64. 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tso IF, Rutherford S, Fang Y, Angstadt M, Taylor SF. The “social brain” is highly sensitive to the mere presence of social information: An automated meta-analysis and an independent study. PLOS ONE 2018;13:e0196503. 10.1371/journal.pone.0196503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lamblin M, Murawski C, Whittle S, Fornito A. Social connectedness, mental health and the adolescent brain. Neuroscience & Biobehavioral Reviews 2017;80:57–68. 10.1016/j.neubiorev.2017.05.010. [DOI] [PubMed] [Google Scholar]
- [20].Van Overwalle F Social cognition and the brain: A meta-analysis. Human Brain Mapping 2009;30:829–58. 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage 2011;55:8–23. 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- [22].Baribeau DA, Dupuis A, Paton TA, Hammill C, Scherer SW, Schachar RJ, et al. Structural neuroimaging correlates of social deficits are similar in autism spectrum disorder and attention-deficit/hyperactivity disorder: analysis from the POND Network. Transl Psychiatry 2019;9:72. 10.1038/s41398-019-0382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Krueger AM, Roediger DJ, Mueller BA, Boys CA, Hendrickson TJ, Schumacher MJ, et al. Para-limbic Structural Abnormalities Are Associated With Internalizing Symptoms in Children With Prenatal Alcohol Exposure. Alcoholism: Clinical and Experimental Research 2020;n/a. 10.1111/acer.14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gautam P, Lebel C, Narr KL, Mattson SN, May PA, Adnams CM, et al. Volume changes and brain-behavior relationships in white matter and subcortical gray matter in children with prenatal alcohol exposure. Human Brain Mapping 2015;36:2318–29. 10.1002/hbm.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nardelli A, Lebel C, Rasmussen C, Andrew G, Beaulieu C. Extensive Deep Gray Matter Volume Reductions in Children and Adolescents with Fetal Alcohol Spectrum Disorders. Alcoholism: Clinical and Experimental Research 2011;35:1404–17. 10.1111/j.1530-0277.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- [26].Treit S, Lebel C, Baugh L, Rasmussen C, Andrew G, Beaulieu C. Longitudinal MRI Reveals Altered Trajectory of Brain Development during Childhood and Adolescence in Fetal Alcohol Spectrum Disorders. Journal of Neuroscience 2013;33:10098–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhou D, Rasmussen C, Pei J, Andrew G, Reynolds JN, Beaulieu C. Preserved cortical asymmetry despite thinner cortex in children and adolescents with prenatal alcohol exposure and associated conditions. Human Brain Mapping 2018;39:72–88. 10.1002/hbm.23818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sullivan EV, Moore EM, Lane B, Pohl KM, Riley EP, Pfefferbaum A. Graded Cerebellar Lobular Volume Deficits in Adolescents and Young Adults with Fetal Alcohol Spectrum Disorders (FASD). Cereb Cortex 2020. 10.1093/cercor/bhaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mattson SN, Riley EP, Jernigan TL, Garcia A, Kaneko WM, Ehlers CL, et al. A decrease in the size of the basal ganglia following prenatal alcohol exposure: A preliminary report. Neurotoxicology and Teratology 1994;16:283–9. 10.1016/0892-0362(94)90050-7. [DOI] [PubMed] [Google Scholar]
- [30].Powell J, Lewis PA, Roberts N, Garcia-Finana M, Dunbar RIM. Orbital prefrontal cortex volume predicts social network size: an imaging study of individual differences in humans. Proceedings. Biological Sciences 2012; 279:2157–2162. 10.1098/rspb.2011.2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, Tregallas JR. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry 2006;6:56. 10.1186/1471-244X-6-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- [33].Reynolds CR, Kamphaus RW. Behavior assessment system for children (3rd ed.). Bloomington: NCS Pearson, Inc.; 2015. [Google Scholar]
- [34].Sparrow SS, Cicchetti DV, Saulnier CA. Vineland adaptive behaviors scales, Third edition(Vineland-3).Bloomington: NCS Pearson, Inc.; 2016. [Google Scholar]
- [35].Burd L, Cotsonas-Hassler TM, Martsolf JT, Kerbeshian J. Recognition and management of fetal alcohol syndrome. Neurotoxicology and Teratology 2003;25:681–8. 10.1016/j.ntt.2003.07.020. [DOI] [PubMed] [Google Scholar]
- [36].Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, et al. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. Neuroimage 2018;183:972–84. 10.1016/j.neuroimage.2018.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 2013;80:105–24. 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dale AM, Fischl B, Sereno MI. Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. NeuroImage 1999;9:179–94. 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- [39].Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006;31:968–80. 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- [40].Kurtzer GM, Sochat V, Bauer MW. Singularity: Scientific containers for mobility of compute. PLOS ONE 2017;12:e0177459. 10.1371/journal.pone.0177459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivières S, Jahanshad N, et al. Common genetic variants influence human subcortical brain structures. Nature 2015;520:224–9. 10.1038/nature14101. [DOI] [PMC free article] [PubMed] [Google Scholar]


