Figure 2.
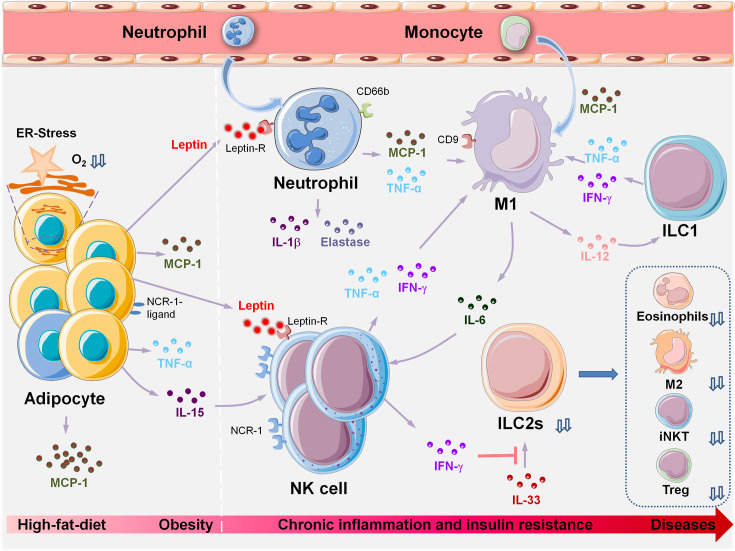
Innate immune cell populations orchestrate metabolic dysfunction in VAT during HFD-induced obesity and associated diseases. HFD-induced obesity leads to adipocyte hypertrophy, pyrolysis, and necrosis. Changes in VAT microenvironment and increased oxygen consumption lead to hypoxia, lipid spillover, and ER stress. Adipocytes and precursor cells produce large amounts of MCP-1 and leptin. MCP-1 induces chemotaxis in a large number of monocytes from peripheral blood, and more than 90% of recruited monocytes locally polarize into M1, which induces and sustains the inflammatory state of VAT. Increased levels of leptin are induced by the inflammatory cytokine TNF-α. Neutrophils, macrophages, NK cells, and activated T and B cells express leptin receptors on their cell surfaces; hence, leptin may be the initial trigger that causes aggregation of these inflammatory cells during obesity, leading to a VAT inflammatory response. The following events occur in the following chronological order: peripheral immune cells (neutrophils, NK) are recruited to the local adipose tissue. Obesity induces upregulation of ligands of NCR-1 on the surface of adipocytes and stimulates NK cell proliferation. Next, monocytes are recruited to the local adipose tissue by MCP-1 and differentiate into M1. IFN-γ can inhibit the reactivity of ILC2s with IL-33, and the deficiency or dysfunction of ILC2s results in insufficient production of IL-4, 5,10,13,33, thus leading to significant decreases in the number of eosinophils, M2, iNKTs and Tregs. VAT, visceral adipose tissue; HFD, high-fat diet; ER-stress, endoplasmic reticulum stress; MCP-1, monocyte chemotactic protein 1; M1, Type I macrophages; NCR-1, NK cell-activating receptor-1; iNKTs, invariant natural killer T cells; Tregs, regulatory T cells; ILCs, innate lymphoid cells.