FIGURE 1.
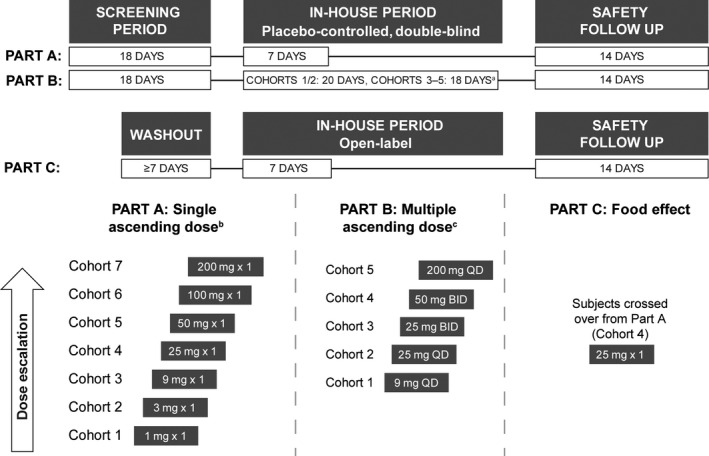
Study design and dose escalation scheme. Eight participants were randomized to each cohort; six to active treatment and two to placebo. Eight participants in Part A, Cohort 4, crossed over to Part C. aThe primary observation period was reduced to 17 days for Cohorts 3–5 of Part B, since available clinical data showed this timeframe provided sufficient safety and tolerability monitoring; bSingle‐dose cohort escalation (Part A): safety data from a single dose plus 1 week in‐house; cMultiple‐dose cohort escalation (Part B): safety data from 2 weeks of treatment until the end of the in‐house period. BID, twice daily; QD, once daily
