Figure 3.
Analysis of flow cytometry data
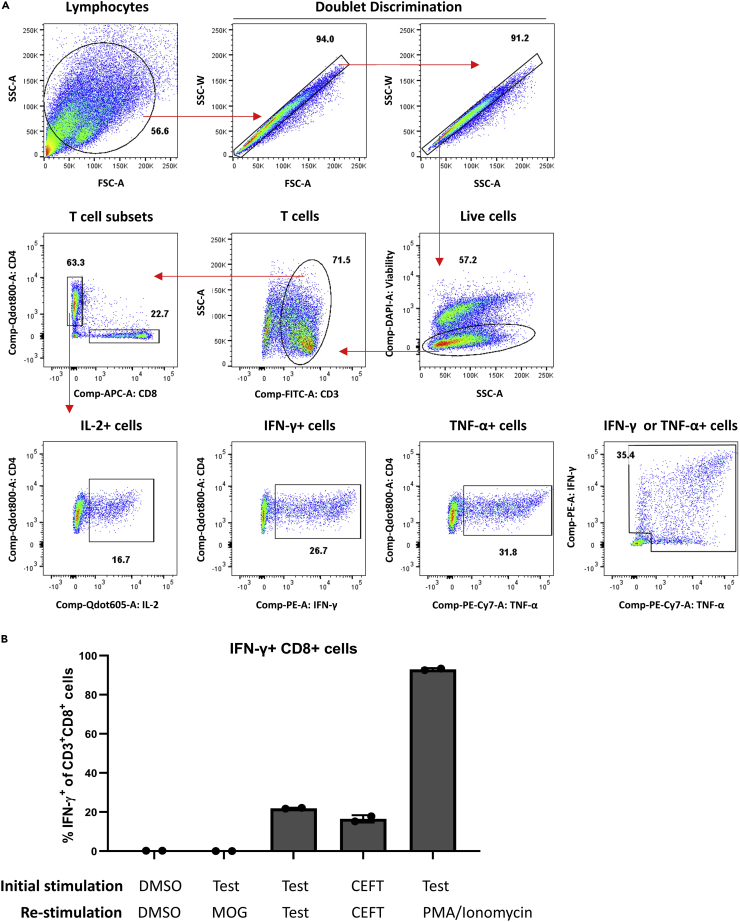
(A) Example gating strategy used to analyze flow cytometry data acquired at the end of the assay. Red arrows indicate gating hierarchy in which T cells are identified as CD3+, live, single cells. T cells are further divided into CD4+ and CD8+ subsets. Expression of effector cytokines, namely IL-2, IFN-γ, and TNF-α, is analyzed under CD4+ T cell gate. Numbers on each graph indicate the frequency (%) of gated events.
(B) Representative results demonstrating detection of reactive T cells following expansion using the described T cell assay protocol. PBMCs from a healthy donor were expanded following stimulation with the vehicle control DMSO, CEFT, which contains known viral epitopes, and test peptides, a pool of five 15mer overlapping peptides spanning mutated SLC35F5 (AKISFFFALCGFWQICHIKKHFQTHKLL) as follows: 1) AKISFFFALCGFWQI, 2) FFFALCGFWQICHIK, 3) LCGFWQICHIKKHFQ, 4) FWQICHIKKHFQTHK, 5) QICHIKKHFQTHKLL (as reported in Roudko et al., 2020). Expanded cells were re-stimulated as indicated with either DMSO, myelin-oligodendrocyte glycoprotein (MOG), which is a self-protein and used as a negative control, test peptides, CEFT, and PMA/Ionomycin, used as positive controls. Percentage of IFN-γ+ CD8 T cells was measured by flow cytometry. Data were represented as mean ± standard deviation (SD).