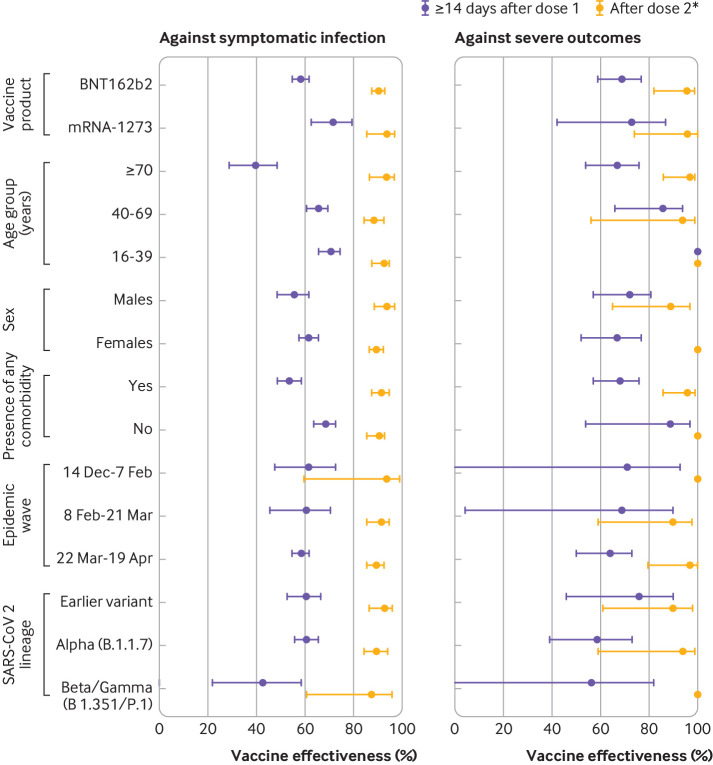
Fig 4.
Adjusted vaccine effectiveness estimates ≥14 days after dose 1 (for those who received only one dose) and ≥0 days after dose 2 of covid-19 mRNA vaccines (BNT162b2, mRNA-1273) by various factors, including vaccine product, patient characteristics, epidemic wave, and SARS-CoV-2 lineage against laboratory confirmed symptomatic SARS-CoV-2 infection and severe outcomes (hospital admission or death) between 14 December 2020 and 19 April 2021 in Ontario, Canada. Models were adjusted for age, sex, public health unit region, biweekly period of test, number of SARS-CoV-2 tests in the three months before 14 December 2020, presence of any comorbidity increasing the risk of severe covid-19, receipt of influenza vaccination in current or previous influenza season, and fifths of neighbourhood level household income, number of people living in each dwelling, proportion of people employed as non-health essential workers, and self-identified visible minority (unless adjusted variable was used for stratification). *For vaccine effectiveness estimates against symptomatic SARS-CoV-2 infection, this interval was ≥7 days after dose 2. Against severe outcomes, vaccine effectiveness was evaluated for the entire period (≥0 days) after receipt of the second dose owing to the small number of outcomes. For subgroup analyses by characteristic and SARS-CoV-2 lineage, individuals vaccinated with either mRNA vaccine were included