Abstract
Background
Cocaine addiction is associated with altered sensitivity to natural reinforcers and intense drug craving. However, previous findings on reward-related responses were mixed, and few studies have examined whether reward responses relate to tonic cocaine craving.
Methods
We combined functional magnetic resonance imaging and a monetary incentive delay task to investigate these issues. Imaging data were processed with published routines, and the results were evaluated with a corrected threshold. We compared reward responses of 50 cocaine-dependent individuals (CDs) and 45 healthy controls (HCs) for the ventral striatum (VS) and the whole brain. We also examined the regional responses in association with tonic cocaine craving, as assessed by the Cocaine Craving Questionnaire (CCQ) in CDs. We performed mediation analyses to evaluate the relationship between regional responses, CCQ score, and recent cocaine use.
Results
The VS showed higher activation to large as compared with small or no wins, but this reward-related activity did not differ between CDs and HCs. The precentral gyrus (PCG), anterior insula, and supplementary motor area showed higher activation during large vs no wins in positive correlation with the CCQ score in CDs. Mediation analyses suggested that days of cocaine use in the prior month contributed to higher CCQ scores and, in turn, PCG reward responses.
Conclusions
The results highlight a unique relationship between reward responses of the primary motor cortex, tonic cocaine craving, and recent cocaine use. The motor cortex may partake in the cognitive motor processes critical to drug-seeking behavior in addicted individuals.
Keywords: cocaine dependence, MIDT, fMRI, motor cortex, ventral striatum
Significance Statement.
Money is a powerful secondary reinforcer, and cocaine addiction is associated with altered sensitivity to reward and intense drug craving. However, it is still unclear whether reward responses relate to tonic cocaine craving. We employed brain imaging to investigate the relationship between cocaine craving and brain responses in a monetary incentive delay task using whole-brain regression analysis. The precentral gyrus, anterior insula, and supplementary motor area showed higher activation during large vs no wins in positive correlation with the cocaine craving score in cocaine users. Mediation analyses suggested that days of cocaine use contributed to higher cocaine-craving scores and, in turn, precentral responses to monetary reinforcer. The results highlight a unique role of the motor cortex in individual motivation to obtain monetary reward. The motor cortex may partake in the cognitive motor processes critical to drug-seeking behavior in addicted individuals.
Introduction
Cocaine is a psychostimulant with powerful addictive properties. Withdrawal from cocaine produces intense craving potentially as a result of downregulated dopaminergic signaling (Volkow et al., 2006), and studies have suggested that cocaine-dependent individuals (CDs) are less motivated for non-drug, including primary, reinforcers (Goldstein et al., 2007, 2010; Tobler et al., 2016). On the other hand, imaging studies have not consistently demonstrated under-responsiveness of the reward circuit, including the ventral striatum (VS), to natural reinforcers in drug users (Garavan et al., 2000; Zhang et al., 2019). We examined in a recent work how CDs responded to food vs cocaine cues and whether CDs responded to food cues differently compared with HCs (Zhang et al., 2019). At a corrected threshold, CDs demonstrated higher activation to cocaine compared with food cues in the hypothalamus, and CDs compared with HC also demonstrated higher hypothalamic activation to food cues. In the same study, with region of interest (ROI) analysis, the VS similarly showed higher response to cocaine than to food cues in CDs and higher food cue responses in CDs than in HCs. The latter findings suggested higher saliency of cocaine cues but did not appear to support the proposition of an under-responsive reward circuit to natural reinforcers in CDs.
Money is a powerful secondary reinforcer and may represent a surrogate of drug for individuals with substance use disorders. Studies have suggested that, although less motivated to maximize hypothetical earning, CDs were more motivated to earn real money than controls (Vadhan et al., 2009; Hulka et al., 2014). For instance, CDs were observed to expend more time and effort completing a gambling task with real money at stake (Vadhan et al., 2009). An earlier study demonstrated that CDs self-administered significantly less cocaine when money (vs. merchandise) vouchers were available as an alternative reinforcer (Hart et al., 2000). In fact, just increases in the chances to win money in a bingo game would be sufficient to counteract participants’ choice to smoke cocaine (Vosburg et al., 2010). Thus, monetary reinforcer is highly salient and the motivation to earn money may be associated with individuals’ state of drug craving in cocaine addiction.
Imaging studies have examined neural responses to monetary reinforcers and showed a mixture of results, including increases, decreases, or no differences in reward-related activations among CDs, relative to HCs (Goldstein et al., 2007; Jia et al., 2011; Patel et al., 2013; Bustamante et al., 2014; Balodis et al., 2016; Konova et al., 2016; Rosell-Negre et al., 2016; Rose et al., 2017). Some reported higher activation to monetary reinforcer in the caudate, putamen, insula, hippocampus, or cingulate gyrus in CDs compared with HCs (Jia et al., 2011; Konova et al., 2016; Rose et al., 2017). Others showed decreased response to monetary reinforcer among CDs in the thalamus, habenula, and frontal and prefrontal gyri compared with HCs (Goldstein et al., 2007; Rosell-Negre et al., 2016; Rose et al., 2017). It is not entirely clear what may have accounted for the differences in findings. The discrepancies may reflect the disparity in participants’ clinical profiles, including current cocaine use and treatment status and/or data analytics (e.g., voxel-wise vs ROI analysis).
Studies have also examined how regional brain responses to monetary reinforcer related to craving. The activations of the inferior frontal cortex to monetary reinforcer correlated positively with the differences in craving post- vs pre-scans among CDs (Konova et al., 2016). One study reported greater VS activation during reward receipt in CDs relative to HCs using ROI analysis, with VS activation correlated inversely with self-reported abstinence and percent cocaine-negative urines (Jia et al., 2011). Using the monetary incentive delay task (MIDT), a more recent study observed increased putamen and motor cortex activation during reward anticipation in cocaine- and/or methamphetamine-dependent participants compared with HCs, and, importantly, the increases in task-related activation in the motor circuit were associated with regular use of the stimulants (Just et al., 2019). Together, although implicating different brain structures, these studies associated higher regional responses to monetary reinforcer with more intense craving. However, other studies demonstrated a contrasting picture. Task-elicited cocaine craving correlated negatively with reward magnitude-related activity during both win and loss trials in the habenula (Rose et al., 2017). Thus, largely based on ROI analyses, these studies have provided less than consistent evidence of reward-related responses in association with craving. More work employing voxel-wise analyses is needed to elucidate the relationship between cerebral activation to monetary reinforcer and individuals’ state of cocaine craving.
Here, we examined the neural responses to monetary reinforcer in a sample of 50 current cocaine users with controlled abstinence. We investigated the relationship between cocaine craving and brain responses to monetary reinforcer in the MIDT using whole-brain regression analysis. Further, as the VS, a key hub of the reward circuit, has consistently been shown to respond to wins vs no-wins or losses in the MIDT (Oldham et al., 2018), we examined the VS as a specific ROI. We had three aims. First, we tested whether the VS showed differences in activation to wins and/or losses in CDs compared with HCs and examined whether the VS responses to monetary reinforcer were associated with tonic cocaine craving, as assessed by the Cocaine Craving Questionnaire (CCQ), in CDs. Second, we performed whole-brain analyses to identify reward-related correlates of tonic cocaine craving, as reflected by the CCQ scores. Finally, we employed mediation analysis to evaluate the inter-relationship between regional responses to monetary reinforcer, tonic cocaine craving, and days of cocaine use in the prior month in CDs.
METHODS
Participants, Informed Consent, and Assessments
Ninety-five adults (50 CDs and 45 HCs) participated in this study (Table 1). CDs met the criteria for current cocaine dependence as diagnosed by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (APA, 2005). Recent cocaine use was confirmed by urine toxicology screens. CDs were drug/medication free while staying at the Clinical Neuroscience Research Unit of the Connecticut Mental Health Center for approximately 2 weeks prior to the functional magnetic resonance imaging (fMRI) scans. All participants were physically healthy with no major medical illnesses or current use of prescription medications. None reported having a history of head injury or neurological illness. The Human Investigation Committee at Yale University School of Medicine approved the study procedures, and all participants signed an informed consent prior to study participation. Some of the subjects participated in our earlier studies (Zhang et al., 2019, 2020a, 2020b).
Table 1.
Demographics and clinical measures of the participants
| Characteristic | CD (n = 50) | HC (n = 45) | P value |
|---|---|---|---|
| Age (y) | 44.5 ± 4.1 | 41.8 ± 8.4 | .10a |
| Sex (M/F) | 40/10 | 29/16 | .09b |
| Years of drinking | 27.0 ± 10.0 | 24.0 ± 10.0 | .15a |
| Years of smoking | 17.4 ± 11.8 | 2.2 ± 6.6 | <.001a |
| CCQ score | 39.6 ± 16.6 | N/A | N/A |
| Monthly cocaine use (g, average, prior year) | 29.8 ± 38.2 | N/A | N/A |
| Cocaine amount per use (g, prior month) | 1.4 ± 1.5 | N/A | N/A |
| Days of cocaine use (prior month) | 19.8 ± 8.8 | N/A | N/A |
| Years of cocaine use | 16.5 ± 9.6 | N/A | N/A |
Abbreviations: CCQ, Cocaine Craving Questionnaire; N/A, not applicable. Values are mean ± SD. a2-tailed 2-sample t test. bχ2 test; Age, sex, years of smoking and years of drinking were used as covariates in data analyses.
Cocaine craving was assessed with the CCQ, Brief version (Sussner et al., 2006). All CDs were assessed with the CCQ, Brief version every 2 or 3 days. There was little day to day variation in the CCQ score (mean ± SD of the coefficient of variation = 0.071 ± 0.047). Thus, we used the CCQ score averaged across all assessments during the inpatient stay to index the tonic level of craving.
CDs and HCs did not differ in age or years of alcohol use but showed a trend-level difference in sex composition and a significant difference in years of cigarette smoking. Thus, in examining imaging data for group differences and correlations with tonic craving, we included age, sex, years of drinking, and years of smoking as covariates in additional analyses.
Behavioral Tasks
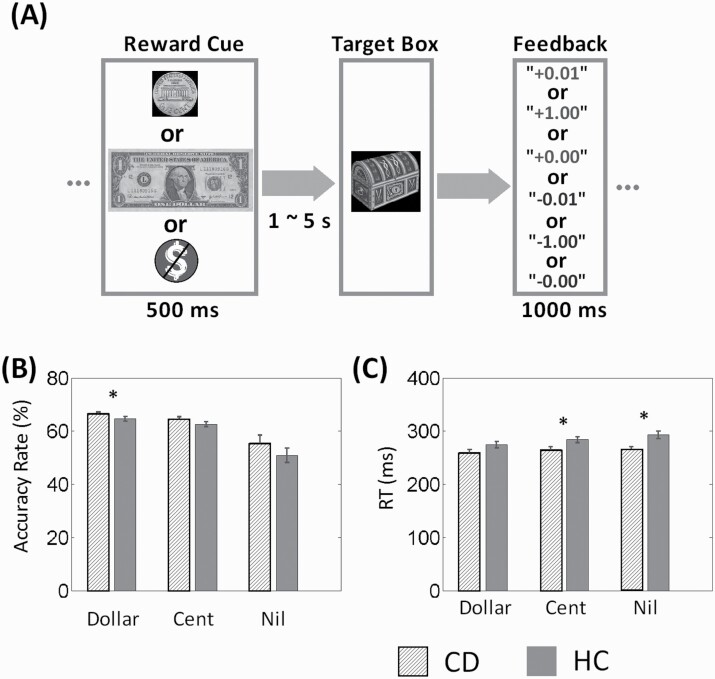
We employed a MIDT as described in our previous studies (Dhingra et al., 2019; 2021) (Figure 1A). Briefly, a reward cue (a dollar, a cent, or no money) appeared on the screen at the beginning of each trial. After a randomized interval (fore-period) between 1 and 5 seconds (uniform distribution), a target box appeared on the screen and disappeared after a short period (response window). Participants were told to press a button as quickly as possible to collect the money in the target box (win) before it disappeared. An accurate trial is defined by a button press before the disappearance of the target box. Otherwise, participants would lose the reward, with the amount deducted from the total win. Participants received the money they won from the task in addition to a base payment. Before the task, participants were instructed that they would receive what they won from the task, and they would win and win more as long as they “played” earnestly in the task.
Figure 1.
Behavioral paradigm and performance. (A) Monetary incentive delay task: a reward cue (a dollar, a cent, or no money) appeared at the beginning of each trial. After a randomized interval between 1 and 5 seconds, a target box appeared on the screen and disappeared after a short period (response window). Participants were told to press the button as quickly as possible to collect the money in the target box (win) before it disappeared. Otherwise, participants would lose the reward, with the amount deducted from the total win. A premature button-press prior to the appearance of the target box terminated the trial and similarly resulted in loss. A feedback window was shown on the screen after each trial to indicate the amount of money won or lost. (B) Accuracy rate and (C) Reaction time (RT) of dollar, cent and no money (nil) trials (mean ± SE). *P < .05.
A premature button press prior to the appearance of the target box terminated the trial and similarly resulted in loss. Feedback was shown on the screen after each trial to indicate the amount of money won or lost. Approximately 42% of all trials were dollar trials, 42% were cent trials, and “no money” constituted the remaining trials. There was an inter-trial interval of 1.5 seconds. The response window started at 300 milliseconds and was stair-cased for each trial type (dollar/cent/no money trials, separately): for instance, if the participant succeeded at 2 successive dollar trials, the window decreased by 30 milliseconds, making it more difficult to win again. Conversely, if a participant failed for 2 successive trials, the response window increased by 30 milliseconds, making it easier to win. We anticipated that the participants would win in approximately 67% each for dollar and cent trials. Each participant completed two 10-minute runs of the task.
Imaging Protocol, Data Preprocessing, and Modeling
Brain images were collected using multiband imaging with a 3-Tesla MR scanner (Siemens Trio, Erlangen, Germany). Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization. Anatomical 3D MPRAGE images were next obtained with spin echo imaging in the axial plane parallel to the AC–PC line with TR = 1900 milliseconds, TE = 2.52 ms, bandwidth = 170 Hz/pixel, field of view = 250 × 250 mm, matrix = 256 × 256, 176 slices with slice thickness = 1 mm and no gap. Functional, blood oxygen level-dependent signals were then acquired with a single-shot gradient echo echoplanar imaging sequence. Fifty-one axial slices parallel to the AC–PC line covering the whole brain were acquired with TR = 1000 milliseconds, TE = 30 milliseconds, bandwidth = 2290 Hz/pixel, flip angle = 62°, field of view = 210 × 210 mm, matrix = 84 × 84, 51 slices with slice thickness = 2.5 mm and no gap, and multiband acceleration factor = 3. Images from the first 10 TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation.
Data were analyzed with Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, University College London, UK). Standard image preprocessing was performed. Images of each participant were first realigned (motion corrected) and corrected for slice timing. A participant whose head motion exceeded 3.0 mm in translation or 3 degrees in rotation was excluded. A mean functional image volume was constructed for each participant per run from the realigned image volumes. These mean images were co-registered with the high-resolution structural image and then segmented for normalization with affine registration followed by nonlinear transformation (Friston et al., 1995; Ashburner and Friston, 1999). The normalization parameters determined for the structure volume were then applied to the corresponding functional image volumes for each participant. Finally, the images were smoothed with a Gaussian kernel of 8 mm at full width at half maximum.
We examined event-related blood oxygen level-dependent signals in a model with 5 trial types distinguished: dollar win, dollar loss, cent win, cent loss, and no money (nil). A statistical analytical design was constructed for each participant using a general linear model with the onsets of “outcome” for each trial convolved with a canonical hemodynamic response function and with the temporal derivatives of the canonical hemodynamic response function and entered as regressors in the model (Friston et al., 1995; Ashburner and Friston, 1999). Realignment parameters in all 6 dimensions were also entered in the model. Serial autocorrelation caused by aliased cardiovascular and respiratory effects was corrected by a first-degree autoregressive model. The general linear model estimated the component of variance explained by each of the regressors.
In group-level or random effects analyses, we performed 1-sample t tests of the whole brain each for CDs and HCs and 2-sample t tests to compare CDs and HCs. To investigate the relationship between reward processing and tonic craving, we conducted whole-brain linear regressions with CCQ score as the regressor. Voxels with peak activity were shown in Montreal Neurological Institute coordinates. For both 1- and 2-sample t tests and linear regressions, we examined a total of 8 contrasts (Supplementary Material).
In ROI analysis, we focused on brain regions from whole-brain linear regressions with CCQ score. We also specifically tested whether the VS showed differences in activation to wins and/or losses in CDs compared with HCs and examined whether VS responses to reward outcomes were associated with tonic cocaine craving, as assessed by the CCQ, in CDs. We employed a VS mask generated by cytoarchitectonic and topographical criteria (Zaborszky et al., 2008) as with our previous studies (Zhang et al., 2017; Zhang and Li, 2018), and computed the β contrast values of the VS and compared CDs and HCs for all the contrasts.
Mediation Analysis
The mediation analysis was performed on only 3 variables that were significantly correlated to each other. In the current study, the CCQ score, number of days of cocaine use in the prior month, and precentral gyrus (PCG) activation were significantly correlated to each other (see Results). Thus, we performed mediation analyses (MacKinnon et al., 2007) using the toolbox M3 (http://wagerlab.colorado.edu/tools) to examine the inter-relationships between PCG activation, tonic cocaine craving, and recent cocaine use (see Results). In a mediation analysis, the relation between the independent variable X and dependent variable Y, i.e., X → Y, is tested to see if it is significantly mediated by a variable M. The mediation test is performed by employing 3 regression equations (MacKinnon et al., 2007):
where a represents X → M, b represents M → Y (controlling for X), c′ represents X → Y (controlling for M), and c represents X → Y. The constants i1, i2, and i3 are the intercepts, and e1, e2, and e3 are the residual errors. In the literature, a, b, c, and c′ were referred as path coefficients or simply paths (MacKinnon et al., 2007; Wager et al., 2008), and we followed this notation. Variable M is said to be a mediator of the correlation X → Y if (c – c′), which is mathematically equivalent to the product of the paths a × b, when it is significantly different from zero (MacKinnon et al., 2007). If the product a × b and the paths a and b are significant, one concludes that X → Y is mediated by M. In addition, if path c′ is not significant, there is no direct connection from X to Y and X → Y is completely mediated by M. Note that path b is the relation between Y and M, controlling for X, and should not be confused with the correlation coefficient between Y and M.
RESULTS
Behavioral Performance
We examined behavioral performance using group (CDs vs HCs) by condition (dollar vs cent) ANOVA (Figure 1). ANOVA of accuracy rate showed a significant group (P = .042) and condition (P = .0001) main effect but not an interaction effect (P = .99). ANOVA of reaction time (RT) also showed a significant group (P = .031) and condition (P = .0002) main effect but not an interaction effect (P = .31). In posthoc analyses, CDs showed higher accuracy during dollar (P = .041) but not cent trials (P = .10), and shorter RT during cent (P = .016) but not dollar trials (P = .072) compared with HCs. CDs but not HCs showed higher accuracy during dollar (P = .026) compared with cent trials (P = .12). Further, in CDs, the CCQ score was negatively correlated with the RT of dollar win trials (r = −0.38, P = .006) as well as the RT of all (dollar + cent) win trials (r = −0.33, P = .02), suggesting a relationship between tonic cocaine craving and eagerness to win.
Regional Activations to Wins, Losses, and Wins vs Losses in the MIDT
The results of one-sample t test of dollar win/loss vs nil, cent win/loss vs nil, and dollar vs cent win/loss are presented in Supplementary Figures 1 and 2 for HCs and CDs. Supplementary Figure 3 showed the results of one-sample t test of dollar win vs dollar loss and cent win vs cent loss. Clusters are summarized in Supplementary Tables 1–3. Please see detailed description in the Supplementary Material.
Specifically, higher activation in VS was observed during dollar and cent wins vs nil in HCs as well as during dollar wins vs nil in CDs. Further, HCs showed higher activation of the dorsal striatum, including the caudate (both head and body), putamen, and globus pallidus during both dollar and cent wins vs losses. CDs showed higher activation of bilateral putamen and caudate body during dollar wins vs losses and of bilateral putamen and globus pallidus during cent wins vs losses. These findings highlight striatal responses in reward win compared with nil or reward loss in both CDs and HCs. However, a two sample t test for each of the 8 contrasts—dollar win/loss vs nil, cent win/loss vs nil, dollar vs cent win/loss, dollar win vs loss, and cent win vs loss—showed that there were no significant differences between CDs and HCs at voxel P < .001 uncorrected and cluster-level P < .05 corrected for familywise error or. Analyses of covariance with age, sex, years of drinking, and years of smoking as covariates also showed no group differences at the same threshold. Likewise, in small volume correction for the VS mask, no voxels showed significant differences between CDs and HCs in any of these contrasts with or without covariates.
Regional Activations to Outcomes in Relation to CCQ Scores in CDs
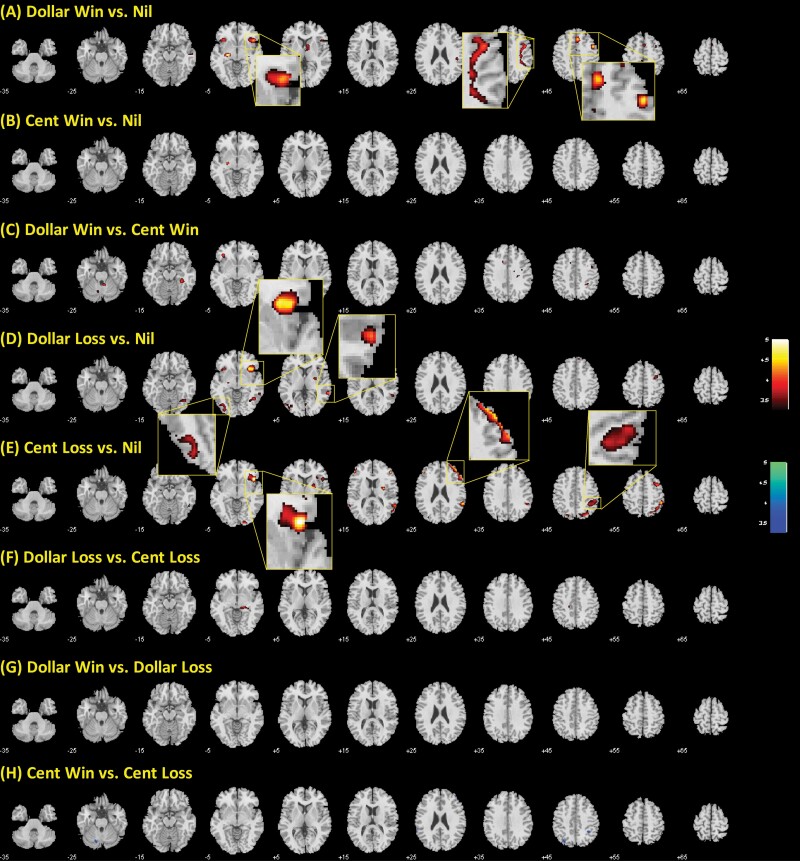
We performed whole-brain linear regression analysis on each of the 8 contrasts against CCQ score in CDs with age, sex, years of drinking, and years of smoking as covariates and reported the findings at voxel P < .001 uncorrected and cluster-level P < .05 FWE-corrected. For the contrast dollar win > nil, the right anterior insula (AI), right PCG, and bilateral supplementary motor area showed activation in positive correlation with CCQ score (Figure 2A). For the dollar loss > nil contrast, the right AI and left occipital gyrus showed activation in positive correlation with CCQ score (Figure 2D). For the cent loss > nil contrast, the right inferior frontal gyrus, AI, and inferior parietal gyrus showed activation in positive correlation with CCQ score (Figure 2E). No brain regions showed a significant correlation with the CCQ score for other contrasts.
Figure 2.
Regional activations to outcomes of (A) dollar win vs nil, (B) cent win vs nil, (C) dollar win vs cent win, (D) dollar loss vs nil, (E) cent loss vs nil, (F) dollar loss vs cent loss, (G) dollar win vs dollar loss, and (H) cent win vs cent loss in correlation with Cocaine Craving Questionnaire (CCQ) at voxel P < .001. Clusters meeting cluster-level P < .05 whole-brain corrected for familywise error of multiple comparisons are highlighted by insets and summarized in Table 2.
Table 2.
Regional activations to outcomes in correlation with CCQ score at voxel P < .001 and cluster-level P < .05 whole-brain corrected for familywise error of multiple comparisons
| Volume (mm3) | Peak voxel | MNI coordinates (mm) | Side | Identified brain region | ||
|---|---|---|---|---|---|---|
| (Z) | x | y | z | |||
| Dollar win > nil (positive correlation with CCQ) | ||||||
| 4509 | 4.82 | 33 | 23 | −11 | R | Insula |
| 7263 | 4.41 | 48 | 5 | 43 | R | Precentral G |
| 3402 | 4.36 | 6 | 26 | 43 | L/R | SMA |
| Dollar win > nil (negative correlation with CCQ) | ||||||
| None | ||||||
| Cent win > nil (positive correlation with CCQ) | ||||||
| None | ||||||
| Cent win > nil (negative correlation with CCQ) | ||||||
| None | ||||||
| Dollar win > cent win (positive correlation with CCQ) | ||||||
| None | ||||||
| Dollar win > cent win (negative correlation with CCQ) | ||||||
| None | ||||||
| Dollar loss > nil (positive correlation with CCQ) | ||||||
| 3672 | 4.49 | 33 | 20 | −6 | R | Insula |
| 4887 | 4.01 | −51 | −73 | 1 | L | Occipital cortex |
| 3294 | 3.84 | 33 | −61 | −11 | R | Occipital cortex |
| Dollar loss > nil (negative correlation with CCQ) | ||||||
| None | ||||||
| Cent loss > nil (positive correlation with CCQ) | ||||||
| 5670 | 5.18 | 51 | 38 | 13 | R | Inferior/middle frontal G |
| 5508 | 4.71 | 45 | 20 | −5 | R | Insula |
| 12 123 | 4.51 | 48 | −43 | 58 | R | Inferior parietal gyrus |
| Cent loss > nil (negative correlation with CCQ) | ||||||
| None | ||||||
| Dollar loss > cent loss (positive correlation with CCQ) | ||||||
| None | ||||||
| Dollar loss > cent loss (negative correlation with CCQ) | ||||||
| None | ||||||
| Dollar win > dollar loss (positive correlation with CCQ) | ||||||
| None | ||||||
| Dollar win > dollar loss (negative correlation with CCQ) | ||||||
| None | ||||||
| Cent win > cent loss (positive correlation with CCQ) | ||||||
| None | ||||||
| Cent win > cent loss (negative correlation with CCQ) | ||||||
| None |
Abbreviations: CCQ, Cocaine Craving Questionnaire; G, gyrus; R/L, right/left; SMA, supplementary motor area.
In small volume correction for the VS mask, we did not observe any voxels with activities in correlation with the CCQ score for any of the 8 contrasts.
VS Activations to Outcome and in Relation to CCQ Scores in CDs
To confirm that CDs and HCs did not differ in VS responses to reward outcome between CDs and HCs and that VS responses did not correlate with CCQ score across CDs, we extracted the β contrast values of the VS for individual participants. We examined VS activity using group (CDs vs HCs) by condition (dollar vs cent compared with nil) by outcome (win vs loss) ANOVA. There were condition (P = .005) and outcome (P = 7.8 × 10–13) but not group (P = .54) differences (supplementary Figure 4A). There was no group by condition (P = .27), group by outcome (P = .89), condition by outcome (P = .07), or group by condition by outcome (P = .63) interaction. In posthoc analyses, CDs did not differ in VS activation of any of the contrasts compared with HCs (all P > .5). Further, VS activity was not correlated with CCQ score for any of the contrasts in linear regressions (all P > .058) (supplementary Figure 4B).
Regional Activations in Relation to Performance in the MIDT
We focused on the brain regions that exhibited a correlation in activity with the CCQ score and examined whether regional responses are related to behavior performance with linear regressions. With the 9 ROIs and 2 behavior measures, we evaluated the results at a corrected threshold P = .05/(9 × 2) = .0028. PCG response to dollar win > nil (β contrast) was positively correlated with the difference in accuracy rate (r = 0.33, P = .021) but not significantly correlated with the difference in RT (r = −0.24, P = .093) of dollar vs nil trials. No other correlations were significant.
To examine whether the PCG response to dollar win vs nil remained correlated with CCQ score after accounting for RT, we performed a linear regression between PCG β estimate (dollar win – nil) and CCQ score with RT (dollar win – nil) as covariates. The results showed that the 2 remained significantly correlated across participants (r = 0.60, P = .0000063).
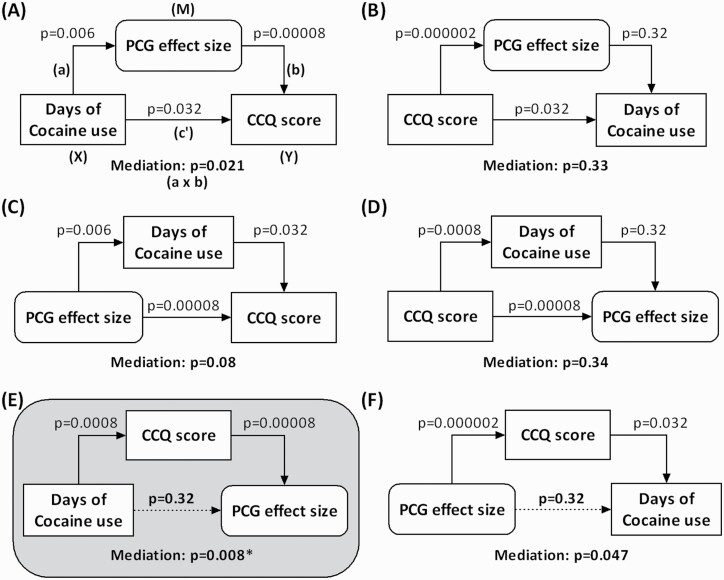
Mediation Analyses
The PCG activation to dollar wins vs nil was positively correlated with the CCQ score as shown earlier. The number of days of cocaine use in the prior month (days of use) was also positively correlated with CCQ score (r = 0.46, P = .0008) and with PCG activation (r = 0.38, P = .0061). Thus, we conducted mediation analysis to examine the relationship between days of use, CCQ, and PCG activation. All 6 models were considered, and the results of mediation were evaluated at a corrected P = .05/6 = .0083. The results showed that only 1 model was significant, showing that days of use contributed to higher tonic cocaine craving and, in turn, PCG activation: days of cocaine use → craving → PCG activity (Figure 3E). With the mediation of CCQ score, days of cocaine use in the past month was not correlated with PCG activation (P = .32), suggesting a complete mediation.
Figure 3.
Mediation analysis of Cocaine Craving Questionnaire (CCQ) score, days of cocaine use, and precentral gyrus activation of dollar win > nil. The P values associated with mediation are for the path a × b (see Methods). Only model E was significant at a corrected threshold P = .05/6 = .0083. The model suggested that tonic cocaine craving mediated the relationship between days of cocaine use and precentral gyrus activation during dollar wins > nil. The variables as descripted in Methods and included in (A) as an example applied to (B-F), too.
Discussion
We investigated the relationship between tonic cocaine craving and regional responses to monetary reinforcer in CDs. The VS showed higher activation to large compared with small or no wins, but this reinforcer-related activity did not differ between CDs and HCs even in ROI analyses. The findings suggest that VS response to monetary wins may not represent a neural marker that defines the under-responsiveness of the reward circuit or relates to tonic craving in cocaine addiction. Further, we observed that the AI showed activity for dollar win vs nil, dollar loss vs nil, and cent loss vs nil in positive correlation with tonic craving in CDs. The data suggest a role of the insula in linking saliency to tonic craving. The right PCG showed activity during dollar vs nil wins in positive correlation with tonic craving. Further, mediation analysis showed that tonic cocaine craving completely mediated the correlation between recent cocaine use and PCG activation. Thus, higher activity of the PCG during monetary wins, as driven by tonic cocaine craving and recent cocaine use, may support individual motivation to obtain monetary reward. Indeed, CDs chose money over acute cocaine administration more frequently when the amount of money offered to them increased in human laboratory (Higgins et al., 1994; Wesley et al., 2014). Although not typically a focus of addiction research, the motor cortex receives dense dopaminergic innervations from the midbrain and may engage in many of the cognitive motor processes during cocaine misuse (Williams and Goldmanrakic, 1993; Hosp and Luft, 2013; Vitrac et al., 2014).
Reward Responses of the VS in Cocaine Addiction
Consistent with the great majority of previous studies discussed earlier, we did not observe a different VS response to rewards between CDs and HCs. In fact, CDs and HCs did not show differences in regional activities for any of the contrasts we examined of the MIDT. As money is a secondary reinforcer, this finding does not necessarily falsify the hypothesis of under-responsiveness to natural reinforcers in cocaine addiction, an issue that can perhaps only be resolved by directly using food and sex as motivating stimuli.
A broader consideration is that dampened dopaminergic signaling as a result of chronic cocaine use may have led to compensatory processes to support neural responses to reward and reward-driven behavior. These compensatory processes may be harnessed to alleviate habitual drug use in cognitive behavioral treatments. On the other hand, behavioral paradigms that employ external stimuli as incentives may not bear on intrinsic motivation, which can be compromised in drug-addicted individuals (Köpetz et al., 2013). For instance, VS activation during reward anticipation in the MIDT was not a significant predictor of resilience in youth with family history of substance use disorders (Martz et al., 2018). Imaging studies of intrinsic motivation appear to engage the reward circuit, including the VS (Daw et al., 2011; Liljeholm and O’Doherty, 2012), and more studies are warranted to examine intrinsic, reward-related processes in CDs.
Reward Responses of the Motor Cortex and Tonic Cocaine Craving
As a part of the primary motor cortex, the PCG is involved in the development of automatized actions as may manifest during habitual cocaine seeking (Hanlon et al., 2011; Ekhtiari et al., 2016; Lench et al., 2017). Although the role of the PCG in craving and other reward-related processes has not received much attention, a meta-analysis of cue reactivity reported higher PCG activation in CDs relative to HCs (Kuhn and Gallinat, 2011). PCG activity in response to cocaine-associated cues predicted cocaine relapse, with relapsers showing higher PCG activity compared with non-relapsers (Kosten et al., 2006). Importantly, examining the neural correlates of CDs and HCs during exposure to film scenes of nature, sexual activity, and cocaine use, a previous study reported higher PCG activity during exposure to cocaine use in CDs compared with HCs and during exposure to cocaine use vs nature in CDs, but not during exposure to cocaine use vs sexual activity in CDs or in HCs (Garavan et al., 2000). The latter findings suggest that PCG activity may be more related to cocaine craving but not arousal. Other studies have implicated the PCG in craving and reward-related processes in pathological gambling and misuse of other substances, as shown in a review of studies of cue reactivity (Yalachkov et al., 2012) and a meta-analysis of reward responses during the MIDT (Luijten et al., 2017). Also of relevance to the current findings were earlier reports of PCG activity during smoking vs neutral cue exposure in positive correlation with Fagerstrom test for nicotine dependence scores in smokers (Smolka et al., 2006). Further, in crystalline-heroin smokers exposed to heroin-related cues, whereas acute cue-elicited craving was associated with activation of the anterior cingulate cortex and other regions in the medial frontal gyrus, tonic craving, as quantified by the Desire for Drug Questionnaire, was associated with activation of the PCG and middle frontal gyrus (Hassani-Abharian et al., 2015). Together, the current findings along with this literature suggest PCG response to monetary reinforcer and drug cues as a useful marker of tonic drug craving.
Cocaine is a strong inhibitor of the dopamine transporter, and chronic cocaine use dampens dopaminergic signaling. Although much of the research of the effects of cocaine on the dopaminergic circuits has focused on the VS, the motor cortex also receives extensive dopaminergic innervations from the midbrain (Gaspar et al., 1991; Raghanti et al., 2016). Previous studies suggested motor performance deficits, elevated motor thresholds, and impaired motor cortical striatal functional connectivity in CDs (Boutros et al., 2001; Hanlon et al., 2010, 2011; Lench et al., 2017). For instance, when performing a finger-tapping task cocaine users made more commission errors and exhibited longer RTs than age- and gender-matched HCs (Hanlon et al., 2010). CDs compared with HCs showed higher resting motor thresholds in transcranial magnetic stimulation of motor cortical regions, suggesting that chronic cocaine use decreased motor cortical excitability (Boutros et al., 2001). CDs showed weaker fronto-striatal coupling during ongoing movement, relative to HCs, in association with prolonged RT (Hanlon et al., 2011; Lench et al., 2017). Further, acute cocaine administration decreased functional connectivity of the PCG with visual cortices during resting state in CDs (Li et al., 2000), although the difference was not observed for resting state without experimental manipulations (Gu et al., 2010). Together, the current and previous studies provide evidence for a role of altered motor cortical activity and connectivity in cocaine addiction.
Insula Activity and Tonic Cocaine Craving
The AI showed higher activations for dollar win > nil, dollar loss > nil, and cent loss > nil in positive correlation with the CCQ score in CDs. The insula plays an important role in signaling saliency and in translating internal bodily states into conscious decision making (Naqvi and Bechara, 2009). Brain imaging studies have consistently shown that cocaine, cigarette, alcohol, and heroin cues activate the insula and that insula cue activity correlates with subjective craving (Naqvi and Bechara, 2009). Human smokers with insula damage were much more likely to be successful than those with brain damage not involving the insula in smoking cessation, with many of them reporting the lack of urge to smoke (Naqvi et al., 2007). In studies of the MIDT, an earlier work found higher insula activation in CDs relative to HCs during successful vs unsuccessful outcomes (Jia et al., 2011), but other studies failed to report a difference (Patel et al., 2013; Bustamante et al., 2014; Rose et al., 2017). Here, we did not observe a significant difference in insula activation between CDs and HCs; however, in CDs, insula showed higher activities during dollar win or dollar/cent loss compared with nil trials in correlation with CCQ score. Altogether, the data suggest a role of the insula in linking saliency to tonic craving.
LIMITATIONS AND CONCLUSIONS
A number of limitations need to be considered for the study. First, we did not observe significant group differences in the reward-related activities, which may reflect the abstinence state of the CDs. An earlier study also examined CDs in the abstinence state and did not find group differences in whole-brain voxel-wise analysis (Bustamante et al., 2014). In contrast, studies of CDs without controlled abstinence tended to reveal group differences, although the findings varied across studies (Goldstein et al., 2007; Jia et al., 2011; Konova et al., 2016; Rosell-Negre et al., 2016). Nonetheless, the lack of group differences in the reward-related activities needs to be replicated in a larger sample. Second, CDs and HCs showed significant difference in years of smoking. Smokers were allowed to smoke if they chose to until the fMRI scan to minimize the effect of nicotine craving, and we included years of smoking and other clinical variables as covariates in data analyses. Nonetheless, we could not entirely rule out the effects of cigarette smoking on the current findings. Further, we did not formulate specific hypotheses as to how reward-related regional responses may differentiate CDs and HCs or associate with craving in CDs. Thus, the study should be considered as exploratory in nature.
In conclusion, cocaine-addicted individuals did not show differences in regional, including ventral striatal, responses to reward outcomes in the MIDT compared with drug-free HCs. Cocaine-addicted individuals showed higher insula and motor cortical responses to monetary reinforcer in association with tonic cocaine craving. Recent cocaine use may result in a higher level of cocaine craving during abstinence, which in turn leads to higher motor cortical activities, reflecting motor readiness to seek external reward. These findings add to the imaging literature by highlighting a neural marker of recent cocaine use. Future studies may examine potentially differentiable neural responses elicited by different types of reinforcer (e.g., money vs food) and whether or how these neural responses may predict cocaine use behavior or treatment outcomes.
Supplementary Material
Acknowledgments
We thank the medical and nursing staff at the Clinical Neuroscience Research Unit, Connecticut Mental Health Center for medical care of the participants.
Supported by NIH grants DA040032, DA045743, DA044749, and DA023248, as well as the Department of Mental Health and Addiction Services (DMHAS) of the State of Connecticut. The funding agencies otherwise have no roles in the conceptualization of the study, data collection and analysis, or the decision to publish these results.
Statement of Interest
The authors declare that they have no conflict of interest.
References
- APA (2005) Diagnostic and statistical manual of mental disorders-IV-TR. Washington DC: American Psychiatric Association. [Google Scholar]
- Ashburner J, Friston KJ (1999) Nonlinear spatial normalization using basis functions. Hum Brain Mapp 7:254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Carroll KM, Potenza MN (2016) Neurofunctional reward processing changes in cocaine dependence during recovery. Neuropsychopharmacology 41:2112–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros NN, Lisanby SH, Tokuno H, Torello MW, Campbell D, Berman R, Malison R, Krystal JH, Kosten T (2001) Elevated motor threshold in drug-free, cocaine-dependent patients assessed with transcranial magnetic stimulation. Biol Psychiatry 49:369–373. [DOI] [PubMed] [Google Scholar]
- Bustamante JC, Barrós-Loscertales A, Costumero V, Fuentes-Claramonte P, Rosell-Negre P, Ventura-Campos N, Llopis JJ, Ávila C (2014) Abstinence duration modulates striatal functioning during monetary reward processing in cocaine patients. Addict Biol 19:885–894. [DOI] [PubMed] [Google Scholar]
- Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ (2011) Model-based influences on humans’ choices and striatal prediction errors. Neuron 69:1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra I, Zhang S, Zhornitsky S, Le TM, Wang W, Chao HH, Levy I, Li CR (2019) The effects of age on reward magnitude processing in the monetary incentive delay task. Neuroimage 207:116368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra I, Zhang S, Zhornitsky S, Wang W, Le TM, Li CR (2021) Sex differences in neural responses to reward and the influences of individual reward and punishment sensitivity. BMC Neurosci. 22:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekhtiari H, Nasseri P, Yavari F, Mokri A, Monterosso J (2016) Neuroscience of drug craving for addiction medicine: from circuits to therapies. Prog Brain Res 223:115–141. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R (1995) Analysis of fMRI time-series revisited. Neuroimage 2:45–53. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA (2000) Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 157:1789–1798. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Duyckaerts C, Alvarez C, Javoy-Agid F, Berger B (1991) Alterations of dopaminergic and noradrenergic innervations in motor cortex in Parkinson’s disease. Ann Neurol 30:365–374. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND (2007) Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry 164:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Moeller SJ, Telang F, Jayne M, Wong C, Wang GJ, Fowler JS, Volkow ND (2010) Liking and wanting of drug and non-drug rewards in active cocaine users: the STRAP-R questionnaire. J Psychopharmacol 24:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y (2010) Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage 53:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Roth AJ, Miller MD, Porrino LJ (2010) Loss of laterality in chronic cocaine users: an fMRI investigation of sensorimotor control. Psychiatry Res 181:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Stapleton JR, Laurienti PJ, Porrino LJ (2011) The association between frontal-striatal connectivity and sensorimotor control in cocaine users. Drug Alcohol Depend 115:240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Foltin RW, Fischman MW (2000) Alternative reinforcers differentially modify cocaine self-administration by humans. Behav Pharmacol 11:87–91. [DOI] [PubMed] [Google Scholar]
- Hassani-Abharian P, Ganjgahi H, Tabatabaei-Jafari H, Oghabian MA, Mokri A, Ekhtiari H (2015) Exploring neural correlates of different dimensions in drug craving self-reports among heroin dependents. Basic Clin Neurosci 6:271–284. [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Bickel WK, Hughes JR (1994) Influence of an alternative reinforcer on human cocaine self-administration. Life Sci 55:179–187. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Luft AR (2013) Dopaminergic meso-cortical projections to m1: role in motor learning and motor cortex plasticity. Front Neurol 4:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulka LM, Eisenegger C, Preller KH, Vonmoos M, Jenni D, Bendrick K, Baumgartner MR, Seifritz E, Quednow BB (2014) Altered social and non-social decision-making in recreational and dependent cocaine users. Psychol Med 44:1015–1028. [DOI] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN (2011) An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry 70:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just AL, Meng C, Smith DG, Bullmore ET, Robbins TW, Ersche KD (2019) Effects of familial risk and stimulant drug use on the anticipation of monetary reward: an fMRI study. Transl Psychiatry 9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Parvaz MA, Froböse MI, Alia-Klein N, Goldstein RZ (2016) Converging effects of cocaine addiction and sex on neural responses to monetary rewards. Psychiatry Res Neuroimaging 248:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köpetz CE, Lejuez CW, Wiers RW, Kruglanski AW (2013) Motivation and self-regulation in addiction: a call for convergence. Perspect Psychol Sci 8:3–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE (2006) Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology 31:644–650. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J (2011) Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci 33:1318–1326. [DOI] [PubMed] [Google Scholar]
- Lench DH, DeVries W, Hanlon CA (2017) The effect of task difficulty on motor performance and frontal-striatal connectivity in cocaine users. Drug Alcohol Depend 173:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, Salmeron BJ, Stein EA (2000) Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med 43:45–51. [DOI] [PubMed] [Google Scholar]
- Liljeholm M, O’Doherty JP (2012) Contributions of the striatum to learning, motivation, and performance: an associative account. Trends Cogn Sci 16:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Schellekens AF, Kühn S, Machielse MW, Sescousse G (2017) Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry 74:387–398. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS (2007) Mediation analysis. Annu Rev Psychol 58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz ME, Zucker RA, Schulenberg JE, Heitzeg MM (2018) Psychosocial and neural indicators of resilience among youth with a family history of substance use disorder. Drug Alcohol Depend 185:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A (2009) The hidden island of addiction: the insula. Trends Neurosci 32:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007) Damage to the insula disrupts addiction to cigarette smoking. Science 315:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Murawski C, Fornito A, Youssef G, Yücel M, Lorenzetti V (2018) The anticipation and outcome phases of reward and loss processing: a neuroimaging meta-analysis of the monetary incentive delay task. Hum Brain Mapp 39:3398–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KT, Stevens MC, Meda SA, Muska C, Thomas AD, Potenza MN, Pearlson GD (2013) Robust changes in reward circuitry during reward loss in current and former cocaine users during performance of a monetary incentive delay task. Biol Psychiatry 74:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti MA, Edler MK, Stephenson AR, Wilson LJ, Hopkins WD, Ely JJ, Erwin JM, Jacobs B, Hof PR, Sherwood CC (2016) Human-specific increase of dopaminergic innervation in a striatal region associated with speech and language: a comparative analysis of the primate basal ganglia. J Comp Neurol 524:2117–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose EJ, Salmeron BJ, Ross TJ, Waltz J, Schweitzer JB, Stein EA (2017) Dissociable effects of cocaine dependence on reward processes: the role of acute cocaine and craving. Neuropsychopharmacology 42:736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell-Negre P, Bustamante JC, Fuentes-Claramonte P, Costumero V, Llopis-Llacer JJ, Barrós-Loscertales A (2016) Reward contingencies improve goal-directed behavior by enhancing posterior brain attentional regions and increasing corticostriatal connectivity in cocaine addicts. PLoS One 11:e0167400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Bühler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF (2006) Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology 184:577–588. [DOI] [PubMed] [Google Scholar]
- Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D (2006) The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend 83:233–237. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Preller KH, Campbell-Meiklejohn DK, Kirschner M, Kraehenmann R, Stämpfli P, Herdener M, Seifritz E, Quednow BB (2016) Shared neural basis of social and non-social reward deficits in chronic cocaine users. Soc Cogn Affect Neurosci 11:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, Haney M, van Gorp WG, Foltin RW (2009) Decision-making in long-term cocaine users: effects of a cash monetary contingency on gambling task performance. Drug Alcohol Depend 102:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitrac C, Péron S, Frappé I, Fernagut PO, Jaber M, Gaillard A, Benoit-Marand M (2014) Dopamine control of pyramidal neuron activity in the primary motor cortex via D2 receptors. Front Neural Circuits 8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C (2006) Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 26:6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Haney M, Rubin E, Foltin RW (2010) Using a novel alternative to drug choice in a human laboratory model of a cocaine binge: a game of chance. Drug Alcohol Depend 110:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008) Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Lohrenz T, Koffarnus MN, McClure SM, De La Garza R 2nd, Salas R, Thompson-Lake DG, Newton TF, Bickel WK, Montague PR (2014) Choosing money over drugs: the neural underpinnings of difficult choice in chronic cocaine users. J Addict 2014:189853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM, Goldmanrakic PS (1993) Characterization of the dopaminergic innervation of the primate frontal-cortex using a dopamine-specific antibody. Cereb Cortex 3:199–222. [DOI] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ (2012) Functional neuroimaging studies in addiction: multisensory drug stimuli and neural cue reactivity. Neurosci Biobehav Rev 36:825–835. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K (2008) Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage 42:1127–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Li CR (2017) Hemispheric lateralization of resting-state functional connectivity of the ventral striatum: an exploratory study. Brain Struct Funct 222:2573–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CR (2018) Ventral striatal dysfunction in cocaine dependence - difference mapping for subregional resting state functional connectivity. Transl Psychiatry 8:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhornitsky S, Angarita GA, Li CR (2020a) Hypothalamic response to cocaine cues and cocaine addiction severity. Addict Biol 25:e12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhornitsky S, Le TM, Li CR (2019) Hypothalamic responses to cocaine and food cues in individuals with cocaine dependence. Int J Neuropsychopharmacol 22:754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhornitsky S, Wang W, Dhingra I, Le TM, Li CR (2020b) Cue-elicited functional connectivity of the periaqueductal gray and tonic cocaine craving. Drug Alcohol Depend 216:108240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.