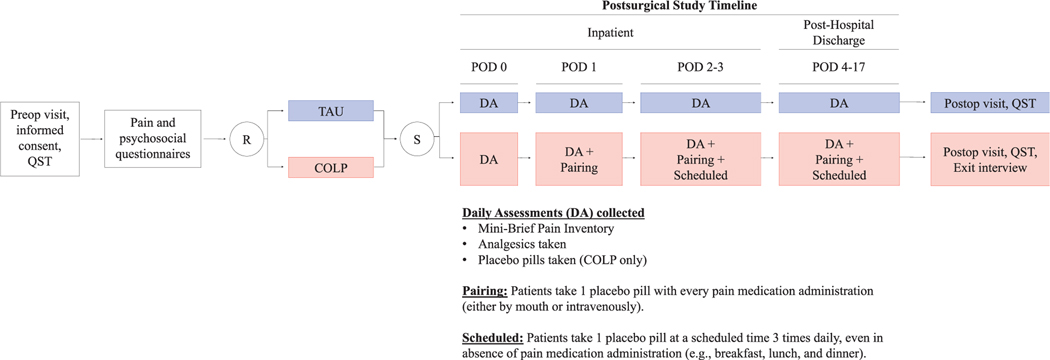
Figure 1.
Patients consenting to participation completed baseline questionnaires and were randomized to either the treatment as usual (TAU) or conditioned open-label placebo (COLP) group. Study staff visited patients in both groups twice daily while inpatient and contacted once daily after hospital discharge to collect daily reports on their analgesics taken and pain scores. Patients in the COLP group also reported how many placebo pills were taken each day. On POD 1, COLP patients began the “pairing” regime by taking one open-label placebo pill each time they took an opioid analgesic. On POD 2, COLP patients were instructed to begin taking 3 daily scheduled placebo pills in addition to the placebo pills paired with each opioid analgesic (“pairing 1 scheduled”). All patients were asked to continue recording daily analgesic consumption and pain scores, with COLP patients also continuing the “pairing 1 scheduled” regime, until their postoperative follow-up appointment (approximately 17 days after surgery). DA, daily assessments; POD, postoperative day; QST, quantitative sensory testing; R, randomization; S, surgery.