TABLE 3.
Representative studies on 3D bioprinting for dental, oral, and craniofacial-related regeneration from 2016 to 2020.
| Bioink | Bioprinting method | Tissue types | Cells/growth factors encapsulated | Key outcomes | Illustration |
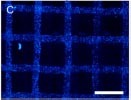
| GelMA | Microextrusion-based | Periodontal complex | PDLCs | The optimized printing conditions supported a high level of PDLCs viability and facilitated cellular proliferation within the construct over 14 days. |
Raveendran et al., 2019 |
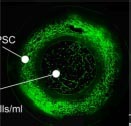
| GelMA | Microextrusion-based | Pulp-dentin complex | hDPSCs + BMP-mimetic peptide | BMP-GelMA bioink formulation provided proper printability and dental specific microenvironment to support hDPSCs high viability, proliferation, and differentiation. |
Park J. H. et al., 2020 |
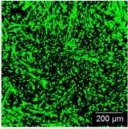
| Dentin-derived ECM + Alginate | Extrusion-based | Pulp-dentin complex | Odontoblast-like cell line (OD21) + acid-soluble dentin molecules | Dentin-derived ECM hybrid cell-laden hydrogel bioink showed high printability and cell survival. This hybrid hydrogel embedded with acid-soluble dentin molecules can enhance odontogenic differentiation. |
Athirasala et al., 2018 |
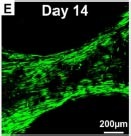
| Fibrinogen + Gelatin + Hyaluronic acid + Glycerol | Custom-made syringe bioprinting | Whole tooth | hDPSCs | A dentin pulp complex with patient-specific shape was successfully produced by co-printing the bio-inks with polycaprolactone. After culturing for 15 days, localized differentiation of hDPSCs in the outer region of the construct was achieved with localized mineralization. |
Han et al., 2019 |
| ECM bioink (2% octapeptide) + AMP | Microvalve bioprinting | Craniomaxillofacial bone tissue | hDPSCs | The cell-laden bioprinted constructs modified with AMP exhibited a high level of mineralization and osteogenic gene expression in vitro and the ECM/1.0AMP composition displayed excellent bone regeneration capability in vivo. |

Dubey et al., 2020 |
| Gelatin-alginate + cellulose nanofibrils + bioactive glass | Extrusion-based | Bone | (i) Human osteoblast-like cells (Saos-2). (ii) hBMSCs | The addition of bioactive glass and cellulose nanofibrils to gelatin–alginate system enhanced their printability and osteogenic activity but resulted in the death of Saos-2 cells due to increased viscosity. |
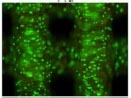
Ojansivu et al., 2019 |
| GelMA + silicate nanoplatelets | Extrusion-based direct-writing bioprinting | Bone | HUVECs + hBMSCs + VEGF | Two GelMA hydrogels containing different concentrations of VEGF were optimized and bioprinted into well-defined 3D architectures, which resulted in the formation of a perfusable lumen, maturation of vascular vessels, and induced osteogenic differentiation. |

Byambaa et al., 2017 |
| Agarose + collagen I | Inkjet | Bone | hBMSCs | Increased solids concentrations of collagen in the 3D-bioprinted hydrogel blend enhanced cell spreading, that ultimately contribute to enhanced and directed MSC osteogenic differentiation. |
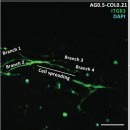
Duarte Campos et al., 2016 |
GelMA, gelatin methacryloyl; PDLCs, periodontal ligament cells; hDPSCs, human dental pulp stem cells; ECM, extracellular matrix; AMP, amorphous magnesium phosphate; hBMSCs, human bone marrow-derived mesenchymal stem cells; HUVECs, human umbilical vein endothelial cells; VEGF, vascular endothelial growth factor; 3D, three-dimensional.
